Waiting longer for children’s second dose: Do benefits outweigh risks?
MANILA, Philippines—Months after the Philippines and other countries began vaccinating children against COVID-19, scientists and experts are now debating and discussing whether extending the interval between two initial doses would boost the young’s immunity.
The country started vaccinating children aged 5 to 11 against SARS-CoV-2, the virus that causes COVID-19, on February 4 in different sites across Metro Manila.
Data from the Department of Education (DepEd) showed that around 14 million basic education students in the same age group are eligible to receive COVID-19 vaccines.
READ: Pediatric vaccination hesitancy by parents: Science provides answers
As of March, the Department of Health (DOH) said a total of 1,233,017 children aged 5 to 11 had been inoculated against COVID-19—around 381,433 of those are already fully vaccinated.
Article continues after this advertisementIn some countries, parents are considering prolonging the interval between doses of vaccines for children—a trick, some said, that might make the vaccines more potent against new COVID variants such as Omicron.
Article continues after this advertisementExtending intervals
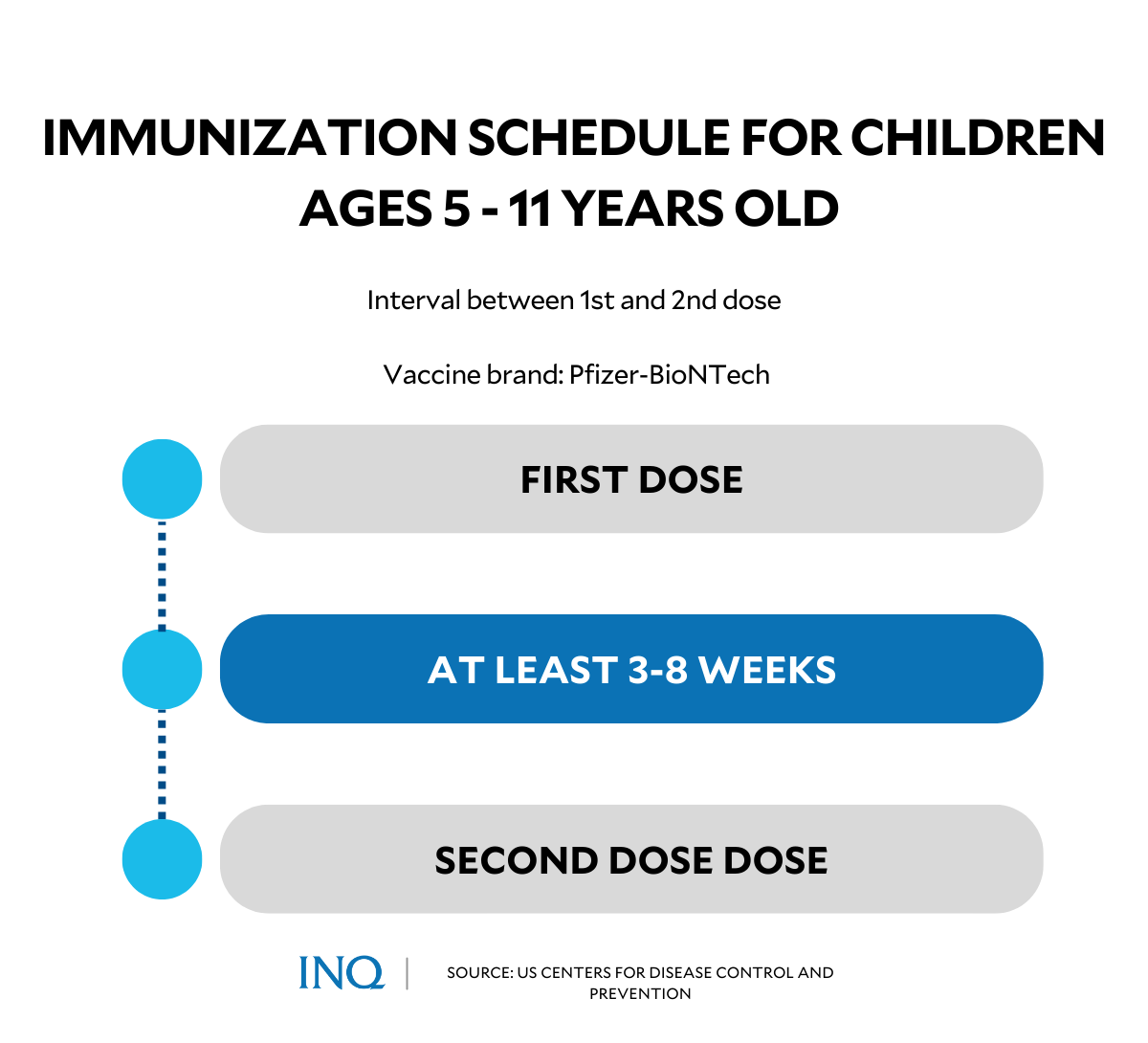
According to the interim guidelines on the management and administration of Pfizer COVID-19 vaccine to pediatric population ages 5 to 11 years old issued by the DOH, the second dose of Pfizer COVID-19 vaccine shall be given three weeks after the first dose to complete the vaccination course.
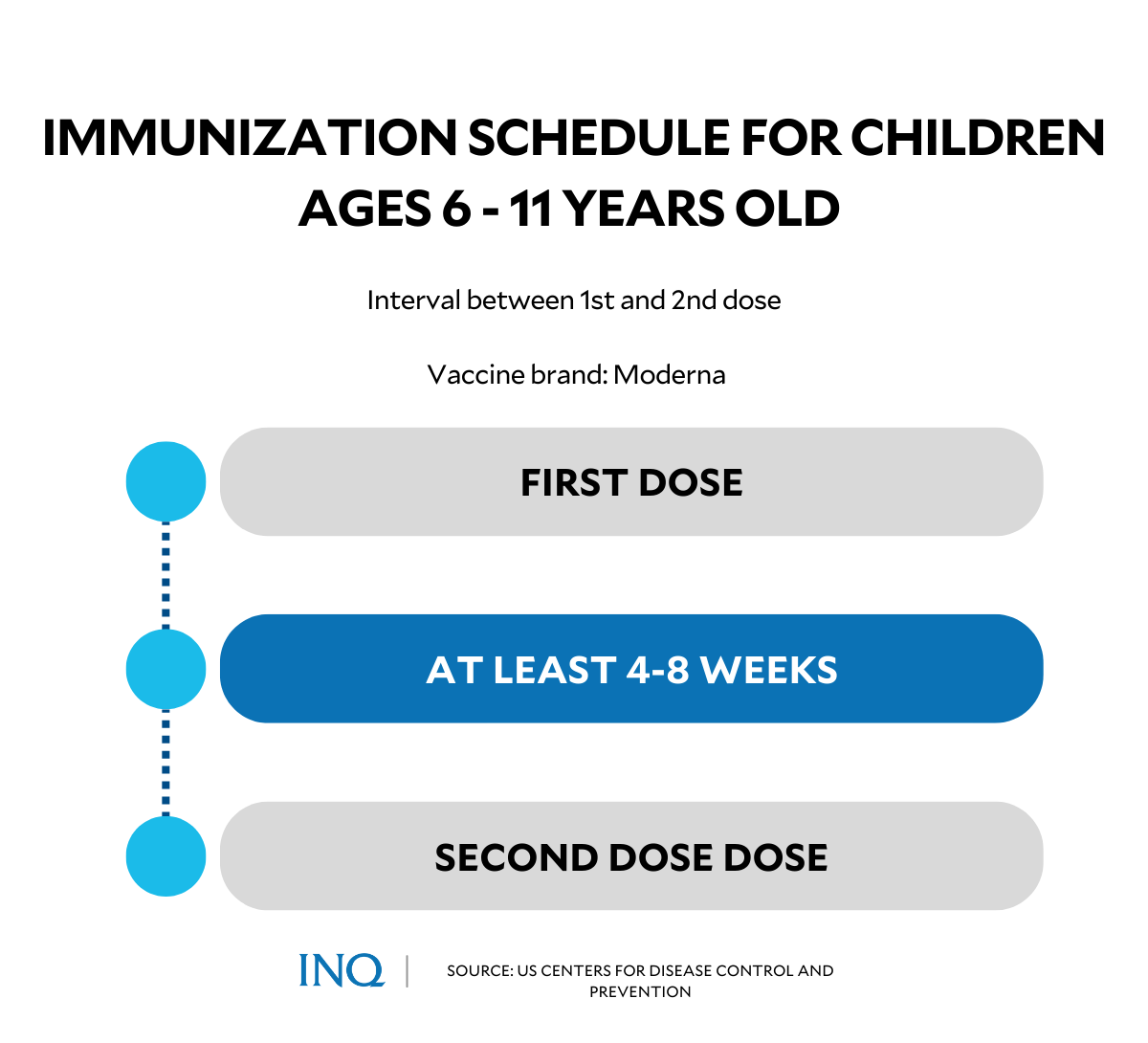
The mRNA vaccine by Moderna—which has been approved for pediatric vaccinations in countries such as the United States and Canada—requires two doses, administered four weeks apart.
The interval recommendations between initial doses for the vaccines can be traced to the initial clinical trials in adults in 2020 at the height of the COVID-19 pandemic.
“Time was lives. There’s no science behind why we picked that three-week or four-week window. It’s pretty arbitrary,” Katelyn Jetelina, an epidemiologist at the University of Texas Health Science Center in Houston, said in an article published in the peer-reviewed scientific journal Nature.
While the three or four-week window between initial doses was the standard, waiting a little longer “makes sense immunologically,” according to the Nature article, since the immune system “needs time to build its defense.”
“The longer the delay before the second injection, the better the immune system will be at recognizing the onslaught and reacting quickly.”
However, amid increases in cases of COVID-19 in many countries paired with the emerging new variants, experts are concerned that extending the interval between doses for children might do more harm.
Some suggest that it is still better to heed the advised three or four-week wait before the second dose and to fully vaccinate young children sooner rather than waiting longer for their second dose.
“You’re delaying the vaccine for a theoretical benefit in the midst of a surge that can do actual harm to children,” said Jessica Snowden, a pediatric infectious-disease specialist at the University of Arkansas for Medical Sciences in Little Rock told Nature.
Small trials on adults
There have been previous studies showing that waiting longer than the recommended three or four weeks before the second vaccine dose might boost immunity and lower the possibility of experiencing side effects.
Others have shown that widening the gap between the first and second vaccine dose could help provide better protection against hospitalization, with one preprint study claiming that it could also increase protection and infection.
Last year, Canada decided to delay the second doses of some adults amid vaccine shortage to increase the proportion of the population with at least one dose. During this period, scientists were able to study the immune response of those who had completed their initial doses three or more months apart and four weeks apart.
Studies found that wider intervals resulted in a greater number of more powerful antibodies.
However, while these studies have shown promising evidence supporting the benefits of longer vaccine intervals, scientists have pointed out that the studies were performed mostly in adults and adolescents, not younger children.
Is it worth the risk?
Some scientists have also emphasized that the immune system of children are not yet fully developed, unlike adults and adolescents.
According to Danuta Skowronski, an epidemiologist at the BC Centre for Disease Control in Vancouver, Canada, the theoretical benefits of waiting longer in between the children’s first and second doses do not outweigh the risk of getting COVID-19.
“We have to remember that these kids aren’t necessarily protected during that gap,” Jetelina said.
Jetelina also noted that “it’s all about risk perception,” adding that some children are not in high-transmission settings.
TSB
For more news about the novel coronavirus click here.
What you need to know about Coronavirus.
For more information on COVID-19, call the DOH Hotline: (02) 86517800 local 1149/1150.
The Inquirer Foundation supports our healthcare frontliners and is still accepting cash donations to be deposited at Banco de Oro (BDO) current account #007960018860 or donate through PayMaya using this link.