Pediatric vaccination hesitancy by parents: Science provides answers

Image: Daniella Marie Agacer
MANILA, Philippines—As children from ages 5 to 11 across the country anxiously waited for their turn to get injected with COVID vaccines, issues raised against the vaccination program slowly plagued the minds of parents and groups.
The vaccination for children aged 5 to 11, which was originally scheduled to start on Feb. 4, had been moved to Feb. 7 in six different sites in Metro Manila.
READ: COVID-19 vaccination for children aged 5-11 to be held in 6 sites
READ: Pediatric COVID-19 vaccination for ages 5 to 11 deferred — DOH
According to data from the Department of Health (DOH), over 168,000 children in that age group have been registered to receive doses of Pfizer vaccine, so far the only vaccine brand approved by the Philippine Food and Drug Administration (FDA) to be used on children.
According to Education Undersecretary Nepomuceno Malaluan, around 14 million basic education students aged 5 to 11 are eligible for COVID vaccination.
READ: 14 millions students aged 5-11 can get COVID-19 vaccination
While preparations were underway for the vaccination of children aged 5 to 11, concerned parents and groups have come out with questions and accusations against the pediatric vaccination program.

Graphic: Ed Lustan
A group’s concerns even reached a court.
In this article, INQUIRER.netwill dissect the causes of hesitancy in vaccinating children, as well as detail opinions from experts and cite findings by scientific studies to address some of the concerns raised by parents and groups on the safety of COVID vaccines.
Petition to stop vaccination
A petition was recently filed at the Quezon City Regional Trial Court to halt the vaccination of children aged 5 to 11 against COVID. It was filed by parents whose children are within that age group.
The parents, in their petition, urged the court to respect “their right to consent (and necessarily, right to refuse consent to COVID-19 vaccination) on behalf of their children and, ultimately, protect life and health of the latter and other children similarly threatened of exposure to undue risks to their life and health.”
Graphic: Daniella Marie Agacer
They also noted that the government is “indirectly forcing” children to receive COVID vaccines, and that parents might become deprived of their right to decide on matters affecting their children.
The petition cited Republic Act No. 11525 which mentioned the “experimental nature” of COVID vaccines in the market.
READ: Court asked to stop COVID-19 vaccination of children aged 5 to 11
The DOH, on Feb. 3, issued a statement on the petition and said that it has recognized the petitioners’ “right to file a case” and that the department will “will wait for the legal process to take its course.”
READ: DOH to push through with pediatric vaccination despite court petition
“However, as far as the national government is concerned, we remain steadfast in our commitment to protect all sectors of society, which include children and other vulnerable groups. As such, we will proceed with the vaccination rollout for the said age group as planned,” the DOH added.
The department also assured that parental forms are required for all minors who will be vaccinated, and that extra measures have been taken to ensure the safety of children.
Concerns piling up
Aside from the group of parents who filed the petition, another group has also recently aired its sentiments about the government’s plan to vaccinate children aged 5 to 11.
The Alliance of Filipinos for Freedom and Informed Choice (AFFIC)—a non-partisan, non-secular, and non-profit organization—has urged the DOH, Department of Education (DepEd), the Department of Social Welfare and Development (DSWD), and the Inter-Agency Task Force on Covid and Infectious Diseases (IATF) to stop the pediatric vaccination program.
Citing RA 11525, the AFFIC alleged that the health department is “blindly pushing for COVID vaccination on children” even though the current vaccinations are “experimental and under Emergency Use Authorization (EUA).”
“Consistent with our mission of advancing children’s protection in the face of COVID 19, we, the Alliance of Filipinos for Freedom and Informed Choice, stand that the vaccination of our children against COVID 19 does not meet the established principle of first ensuring that no harm is done,” the group said in a full page ad in the Inquirer.
“It is a grave act of child rights violation because vaccinating children for COVID-19 exposes them to risks that far outweigh the hoped-for benefit, especially given that children have an extremely low risk from this disease,” it added.

Graphic: Ed Lustan
“There is absolutely no justification to hurry the vaccination and put children in harm’s way at this time.”
The group had asked the government to shelve its plan to inoculate children aged 5 to 11 on the following grounds:
- “The replacement of robust natural immunity of children with a shorter and less competent vaccination immunity is cause for grave concern.”
- “The effects on the maturation of the immune, reproductive and nervous system of infants/young children/teens need to be carefully and scientifically assessed.”
- “The rising incidence of adverse events from the current COVID-19 experimental injections, including myocarditis, cannot simply be ignored and merits investigation;”
- “The COVID vaccination programs and policies have so far been divisive, coercive, anti-poor and discriminatory, affecting not only public health but the nation’s social fabric of solidarity and respect in the face of pandemic, and thus needing a thorough review before commencing another round of divisive vaccination rollout.
It also emphasized that the pharmaceutical industry will not be legally and medically liable for any adverse vaccination effects.
The group, which claimed to be not against the government, also called on the national government—as a signatory to the United Nations Convention on the Rights of the Child (UNCRC)—“to uphold the Filipino children’s right to life, survival, and development.”
“AFFIC is a national alliance that is not against the government. We are engaging the government in demanding accountability in protecting and upholding the rights of the Filipino constituents, especially in protecting the children,” the group said.
Health reform advocate Dr. Tony Leachon, in response to the group’s statement, said vaccination remains the most effective way to prevent infection from SARS-COV-2—the virus that causes COVID-19.
“I know that parents and other concerned citizens are anxious about vaccination for children. But I’m a firm believer of science and ethical regulatory approvals of drugs and vaccines,” Leachon told INQUIRER.net.
Soothing fears with science
The hesitancy of some parents in vaccinating their children against COVID, according to an article published in The Journal of the American Medical Association (JAMA), can be attributed to concerns about the safety and possible side effects of the vaccine.
According to Leachon, reports submitted to the Vaccine Adverse Event Reporting System (VAERS)—a national passive vaccine safety surveillance system in the United States (US)—showed that “approximately 97 percent” of children aged 5 to 11 years who received Pfizer-BioNTech COVID-19 vaccine had ‘non-serious’ side effects.”

Graphic: Ed Lustan
VAERS, which analyzes reports of adverse events after a person has been vaccinated, is jointly managed by the US Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration (FDA).
A study published in the New England Journal of Medicine (NEJM) stated that there have been no serious vaccine-related adverse effects among the 1,517 fully vaccinated children aged 5 to 11 who participated in clinical trials to demonstrate for the Pfizer vaccine.
Evaluation of the BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age
“After a median 2.3-month follow-up, the researchers concluded that BNT162b2 is safe, immunogenic, and effective for the young age group they studied,” said an article published in JAMA, citing the research funded by Pfizer and BioNTech.
The Philippine Pediatric Society (PPS) and the Pediatric Infectious Disease Society of the Philippines (PIDSP), in a recent joint statement, likewise cited published data from random clinical trials for the Pfizer vaccine for children.
The data, the PPS and PIDSP said, “showed that a vaccination regimen consisting of two 10-0-μg (microgram) doses of the vaccine administered 21 days apart among 5–11 year-old had a favorable safety profile and antibody levels comparable to those in 16-to-25-year-olds.”
“A vaccine efficacy of 90.7% (95% CI, 67.7 –98.3) has formed the basis of approval for use of the vaccine in the Philippines and in other countries,” the joint statement stated.
PPS-PIDSP Joint Position Statement on COVID-19 Vaccination for Children ages 5 to 11 years old
Secretary Carlito Galvez Jr., chief implementer of the National Task Force Against COVID-19 (NTF), had also said that the Pfizer vaccine had “a lower formulation appropriate for children.”
According to US CDC, children within the age group 5 to 11 years old will receive “a separate vaccine formulation denoted with an orange cap of the Pfizer-BioNTech COVID-19 vaccine, and will receive the vaccine with a smaller needle.”
Pfizer-BioNTech’s pediatric formulation for the age group contains 10 micrograms per dose, while the adult and adolescent formulation—which has a purple vial cap—contains 30 micrograms per dose.
“The policy on vaccinating children aged five to 11 is the result of careful study by health experts and has been approved in many countries, including the United States of America, which has one of the most stringent regulatory bodies in the world,” the DOH said.
“We would also like to point out that parental consent forms are required for all minors who will be vaccinated, and that extra measures have been taken to ensure their safety,” it added.
Serious adverse events, myocarditis
In December last year, when the Food and Drug Administration (FDA) granted EUA to the Pfizer vaccine for children aged 5 to 11, then FDA director Eric Domingo assured the public of the efficacy of the vaccine.
“Its efficacy rate is high, above 90 percent for kids 5 to 11 years,” he said.
“The adverse events during the clinical trials were very mild like other vaccines that children received. Some experience fever, slight pain in the injection site. But there are no unusual or important safety signals for us not to give this EUA,” he added.
READ: FDA okays use of Pfizer COVID-19 vaccine to children 5-11 years old
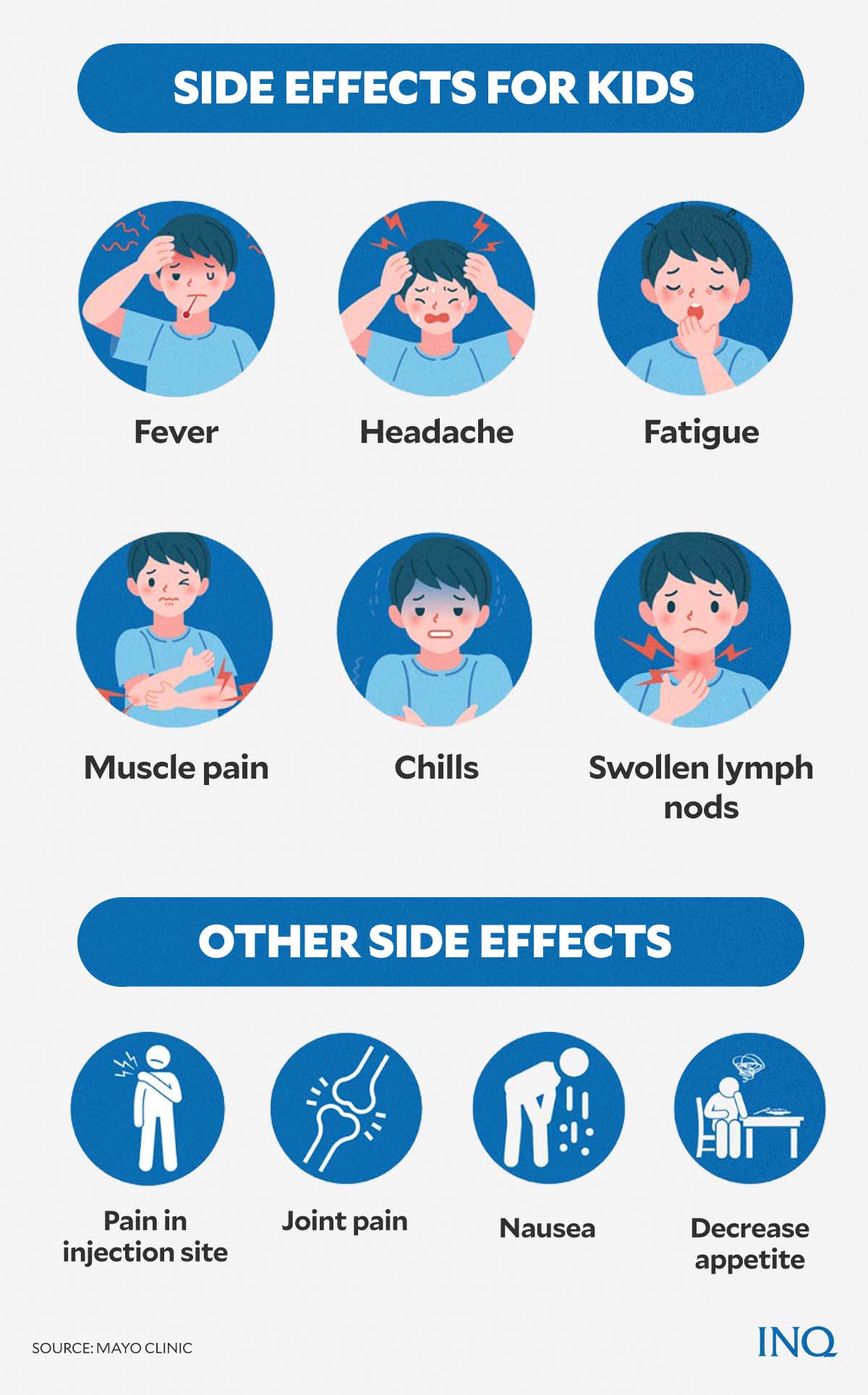
According to Mayo Clinic, a non-profit American academic medical center, some of the commonly reported side effects of Pfizer’s vaccine for children included:
- pain in the arm or injection site
- fatigue
- headache
- chills
- muscle pain
- fever
- joint pain
- swollen lymph nodes
- nausea
- decreased appetite
Among the side effects, according to the US FDA, were non-severe allergic reactions like rashes, itching, hives or swelling of the face. Some of the other side effects were diarrhea, vomiting and fainting.
“Safety data from United States surveillance showed that serious adverse events following Pfizer-BioNTech vaccination in children ages 5–11 years occurred in 2 percent of recipients with fever and vomiting as the most frequently reported,” the PPS and PIDPS said.
The US FDA noted that the aside from the commonly reported side effects, serious and unexpected side effects may occur.
Some of the signs of severe allergic reaction, which would usually occur within a few minutes to an hour after getting a dose of the vaccine, included:
- difficulty breathing
- swelling of the face and throat
- fast heartbeat
- bad rash all over the body
- dizziness and weakness
However, the US FDA clarified that there is a “remote chance” that the vaccine could cause severe allergic reactions among children.
Another serious side effect of the Pfizer vaccine on children aged 5 to 11, according to some concerned parents and groups, are myocarditis and pericarditis.
Myocarditis refers to the inflammation of the heart muscle, while pericarditis is the inflammation of the lining outside the heart.
The US FDA said myocarditis and pericarditis “have occurred in some people who have received the vaccine” and that the “symptoms began showing few days after receiving the second dose of the vaccine.”
But, according to the US FDA, the chance of having myocarditis and pericarditis occurring among children aged 5 to 11 is still very low.
The US CDC likewise explained that, in general, adolescents aged 12 to 17 years have a higher risk for myocarditis than children aged 5 to 11 years.
“Therefore, we are not sure if the cases of myocarditis that occurred after COVID-19 vaccination in adolescents will predict the cases that could occur in children after COVID-19 vaccination,” the US CDC added.
This was echoed by Leachon, who described myocarditis as a “rare and serious adverse event that has been associated with mRNA-based COVID-19 vaccines.”
“Reporting rates for vaccine-associated myocarditis appears highest among males aged 12-19 years. To date, myocarditis among children aged 5-11 years appears rare,” said Leachon.
The US FDA advised parents to seek immediate medical attention if their child experiences chest pain, shortness of breath and feeling of having a fast-beating, fluttering, or pounding heart after receiving a dose of COVID vaccine.
According to the local FDA, around 2,732 reports on the effects of the vaccines on adolescents, who were inoculated from Oct. 15, 2021, to Jan. 16 this year, were submitted.
At least 139 cases were “serious,” 2,563 were “non-serious” and the effects on the rest have not yet been determined.
The most common reactions were dizziness, pain at the site of the injection, fever, headache and high blood pressure.
“There were two cases of reported myocarditis and one case of pericarditis, all of which have resolved, and causal link still needs to be established,” the PPS and PIDSP said, citing the FDA’s weekly overview on suspected adverse reactions to vaccines.
Benefits outweigh risks
Data from the World Health Organization (WHO) said children and younger adolescents between ages 5 to 14 years accounted for only seven percent or 7,058,748 of the reported COVID-19 cases globally, and 0.1 percent, or 1,328, of reported global deaths caused by the disease.
The WHO also noted that children and younger adolescents from the same age group also usually demonstrate fewer and milder symptoms of SARS-CoV-2 infection compared to adults and are less likely than adults to experience severe COVID-19.
So, if children do not frequently experience severe illness with COVID-19, why do they need to receive COVID vaccine?
“If your child gets COVID-19, a COVID vaccine could prevent him or her from becoming severely ill or experiencing short-term or long-term complications,” the Mayo Clinic explained.
“Children with other health conditions, such as obesity, diabetes and asthma, might be at higher risk of serious illness with COVID-19,” it added.
The PPS, PIDSP, and DOH also said vaccinating children aged 5 to 11 can help prevent the spread of the COVID virus.
“Based on an indication from a modeling data stated by the European Center for Disease Prevention and Control, vaccinating children aged 5-11 years old could reduce SARS-CoV2 transmission in the whole population,” said the PPS and PIDSP.
“Vaccinating them is crucial to achieve our goal of protecting all members of the Filipino family-children, adults, and senior citizens,” the DOH said.
“This will enable us to continue safe reopening of schools and other public spaces, as well as ensure the full economic recovery of our nation,” it added.
As the US CDC has said, when it comes to the discussion on whether children aged 5 to 11 should get COVID vaccines, the benefits of vaccination outweigh the known and potential risks.
TSB
For more news about the novel coronavirus click here.
What you need to know about Coronavirus.
For more information on COVID-19, call the DOH Hotline: (02) 86517800 local 1149/1150.
The Inquirer Foundation supports our healthcare frontliners and is still accepting cash donations to be deposited at Banco de Oro (BDO) current account #007960018860 or donate through PayMaya using this link.