PH vaccination drive enters new phase—children, minors

FILE PHOTO: A father with his children aboard their bicycle crosses an almost empty road in Manila on March 20, 2020, after the government imposed an enhanced community quarantine against the rising numbers of coronavirus infections. AFP
MANILA, Philippines—The Philippine vaccination program has entered a new phase—the inoculation of children and young adults.
On Oct. 15, the national government started the pilot run of vaccination for minors with comorbidity against SARS Cov2, the virus that causes COVID-19.
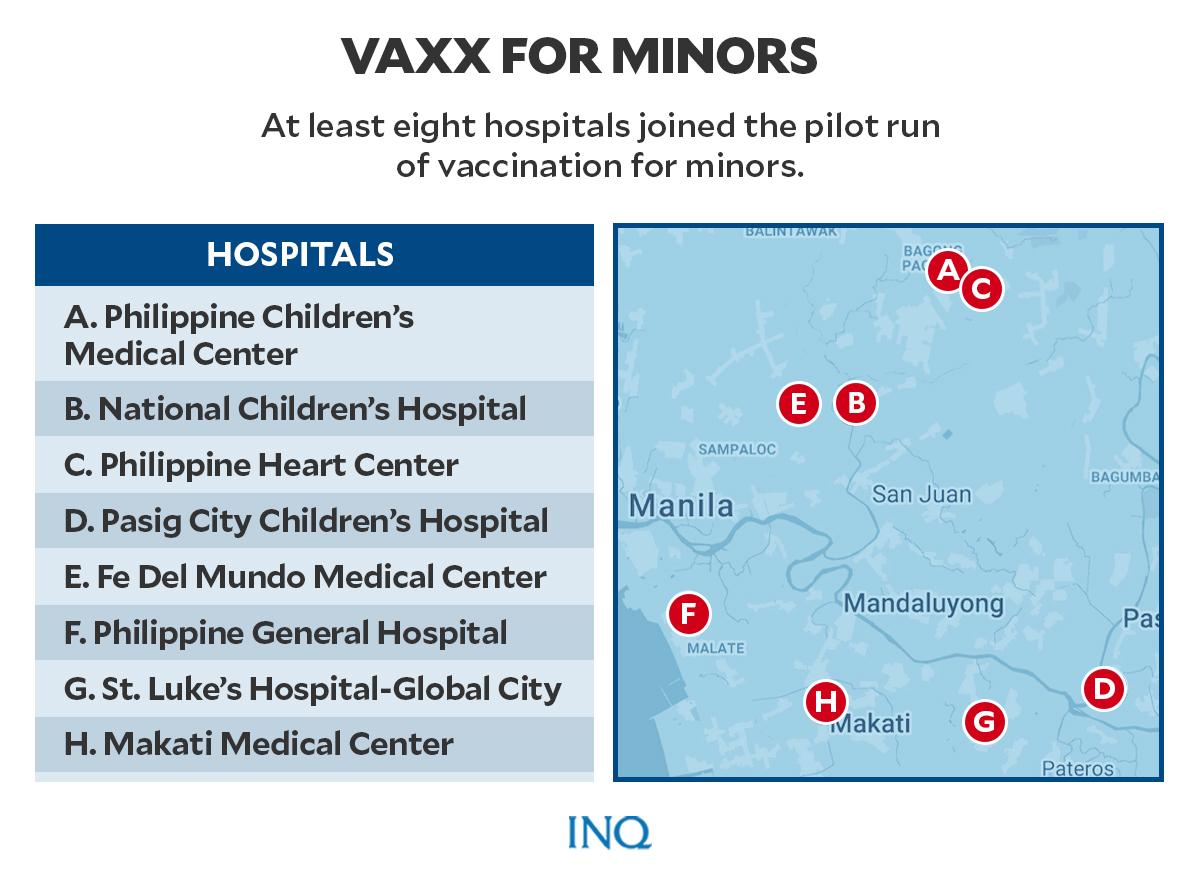
At least eight hospitals took part in the pilot vaccination run. These are Philippine Children’s Medical Center, National Children’s Hospital, Philippine Heart Center, Pasig City Children’s Hospital, Fe Del Mundo Medical Center, Philippine General Hospital, St. Luke’s Hospital-Global City, and Makati Medical Center.
Graphic by Ed Lustan
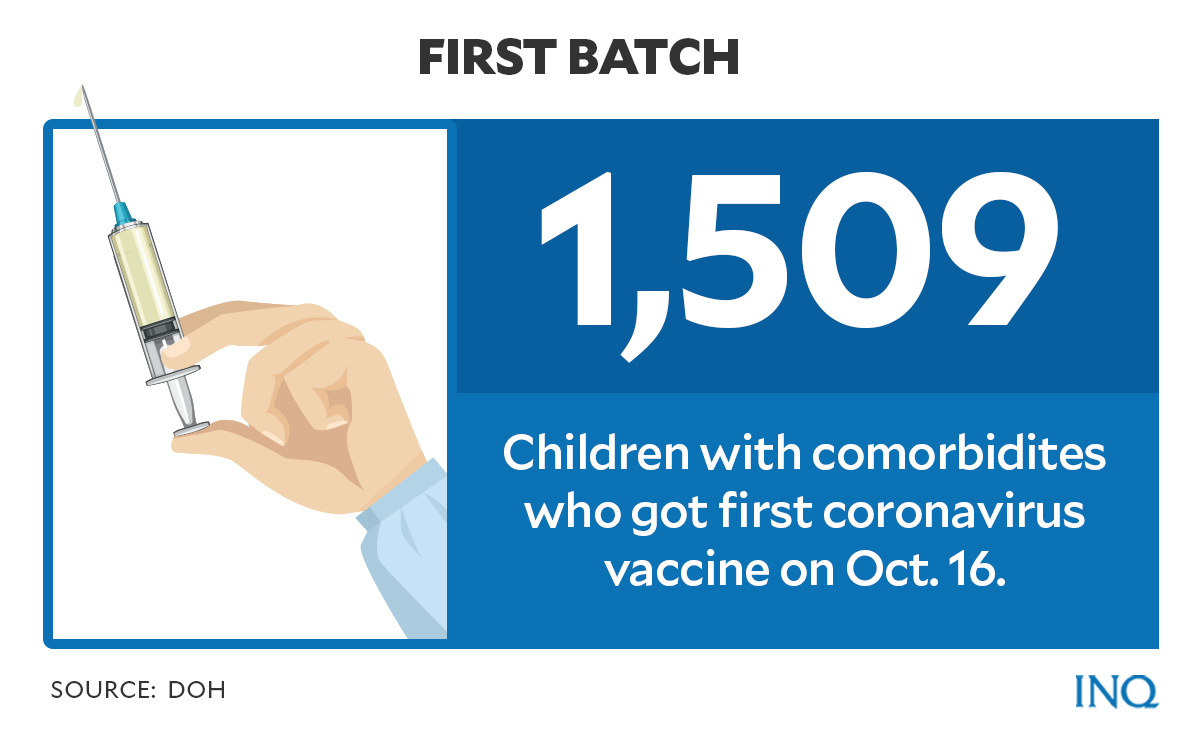
According to the Department of Health (DOH), as of Oct. 16, a total of 1,509 children with comorbidity, or pre-existing health conditions, have received their first vaccine dose.
Some questions about the new phase of the vaccination program have been answered here.
Article continues after this advertisementPediatric COVID vaccination
Who are eligible to register for the pilot run of the pediatric COVID-19 vaccination?
Article continues after this advertisementMinors from ages 12 to 17 who suffer from any of the following comorbidity are considered part of the Pediatric A3 category and are eligible for vaccination:
- Genetic conditions
- Neurologic conditions
- Metabolic/endocrine diseases
- Cardiovascular diseases
- Obesity
- Human immunodeficiency virus infection
- Tuberculosis
- Chronic respiratory diseases
- Renal disorders
- Hepatobiliary (referring to the biliary tract or gall bladder, kidney, liver) diseases
Pediatric patients with medical complexity or those with long-term dependence on technical support and those who are immunocompromised due to disease or treatment are also qualified to receive vaccines for minors.
Graphic by Ed Lustan
Is the pilot run held nationwide?
So far, the vaccination drive for children with comorbidity is carried out only in select hospitals in Metro Manila.
The next phase of the vaccination rollout for minors is scheduled for Oct. 22 at 17 in hospitals identified by local governments in Metro Manila.
Graphic by Ed Lustan
Which vaccine brands will be used for the pilot rollout?
The country’s drug regulators have granted emergency use authorization (EUA) to two vaccine brands —Pfizer and Moderna— for children aged 12 to 15 years old.
READ: PH FDA OKs emergency use of Pfizer’s COVID vaccine for 12 to 15 yrs old
READ: FDA OKs emergency use of Moderna vaccine on adolescents aged 12 to 17
Are there any requirements?
According to DOH, informed consent by the parent or guardian and child prior to vaccination should be obtained. Children with comorbidity are also advised to secure clearance from their pediatricians or trained doctors.
In a statement issued last Sept. 29, the DOH said children with comorbidity will be inoculated in selected sites with clearance from their doctors. If residing in far-flung areas, minors would be injected with clearance from on-site doctors with guidance from a checklist issued by the Pediatric Infectious Disease Society of the Philippines.
“We have to ensure that children have equitable access to vaccines,” the DOH said.
“Eventually, as we get more local experience, we will be able to retool our current vaccinators on the additional precautionary steps on screening and vaccine administration,” it added.
Benefit outweighs risks
The Philippine Society of Allergy, Asthma, and Immunology (PSSAI) in a recent statement told the public that the benefits of COVID vaccines and vaccination outweigh the risks.
“Based on current data the benefits of vaccination to the individual person and community outweigh the risks of adverse reaction to these vaccines,” said PSAAI.
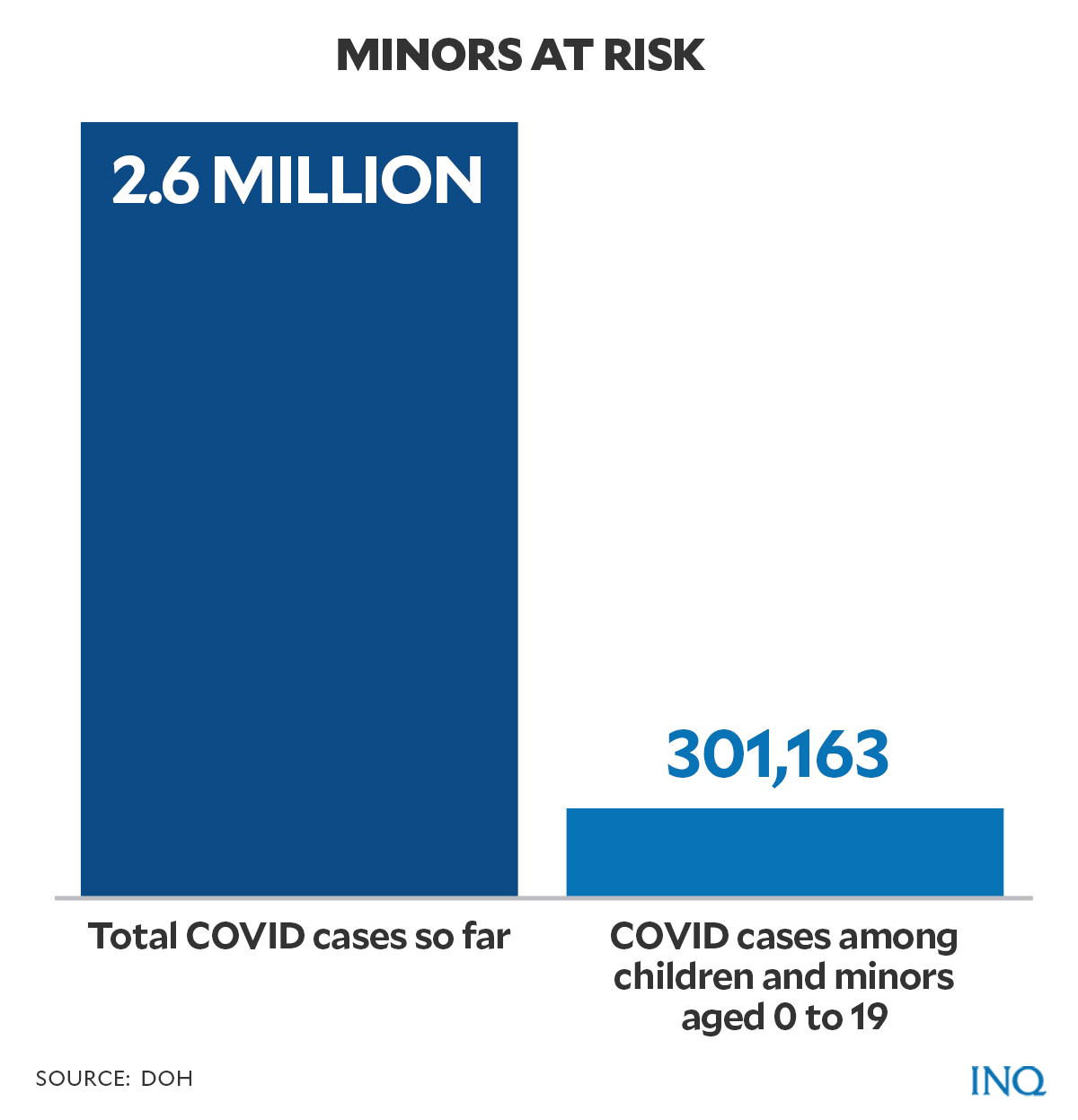
Citing data from DOH, out of the 2,659,758 reported cases of COVID-19 in the country as of Oct. 13, at least 11 percent or 301,163 cases were pediatric cases with patients aged 0-19 years old.
Deaths, according to the organization, were highest among pediatric patients aged 4 and below. It is followed by those in the 15-19 year old age group.
The pilot vaccination runs also come amid the threats brought by the Delta variant, the highly transmissible SARS Cov2 strain which has become predominant worldwide.
Health experts believe that the increase in COVID-19 cases among Filipino children in September could be attributed to Delta.
READ: Alarm bells ring over rising Delta infections among PH kids
Adverse reactions
Health Undersecretary Maria Rosario Vergeire on Monday (Oct. 18) said that there had been only four reports of adverse reactions among inoculated children, most of which were minor cases.
Vergeire said one child experienced high blood pressure which eventually subsided, while two others had stress-related reactions to the vaccine. A minor allergic reaction has also been reported, though it was managed immediately.
Vergeire clarified, however, that these reports of adverse events are not yet official.
“Let me caution all of you. It’s not official yet,” said Vergeire.
READ: 4 reported adverse reactions out of 1,509 pediatric COVID-19 vaccinations — DOH
An updated statement issued by PSAAI listed some of the adverse reactions among adolescents aged 12-17 years old who received doses of mRNA vaccines by Pfizer and Moderna.
Based on post-authorization monitoring of reactions in adolescents who received jabs of mRNA vaccines in the United States (US), reactogenic reactions—both local and systemic—were commonly reported.
Scientist Caroline Hervé and her colleagues explained in their paper that reactogenicity pertains to “a subset of reactions that occur soon after vaccination, and are a physical manifestation of the inflammatory response to vaccination.”
“These symptoms may include pain, redness, swelling or induration for injected vaccines, and systemic symptoms, such as fever, myalgia, headache, or rash,” they added.
Most of the cases cited by PSSAI were mild and usually disappear after a few days.
Local reactogenic reactions usually occurred within two days following the first and second dose. Systemic reactogenic reactions, like fever, were mostly experienced by vaccinated adolescents after their second dose.
Aside from reactogenic reactions, post-authorization monitoring also noted cases of a more specific reaction to mRNA vaccines. Few cases of syncope — fainting, loss of consciousness, or passing out — have been reported as well as myocarditis or inflammation of the heart muscle.
“Syncope, which is separate from anaphylaxis, is a reaction to vaccines almost unique to adolescents and with a female preponderance,” said PSAAI.
“Providers must be able to anticipate and provide support in such a scenario during and post-vaccination,” PSAAI added.
Data from Vaccine Adverse Event Reporting System (VAERS) — a US vaccine safety monitoring system — showed that as of July 16, 13.3 percent of 9,246 (about one in 1,000 vaccines) adverse events, which had been reported to VAERS, involved syncope.
The most common reaction was dizziness at 20.1 percent followed by syncope and headache at 11.1 percent.
On the other hand, myocarditis occurred in some adolescents after receiving a second dose.
“Myocarditis associated with the mRNA vaccines became evident during post-approval safety surveillance of vaccinations and was seen to peak in males aged 12-17 years,” the statement read.
PSAAI emphasized that most reported cases were mild with resolution in 95 percent of cases. The organization also added that chances of getting myocarditis are higher after contracting COVID-19 than post-vaccination.
“As monitoring of these patients continues, no mortality has so far been directly attributed to the vaccine,” said PSAAI.
It said while cases of mortality are rare, children or minors and their guardians “must be familiarized with this risk to aid in timely detection.”
“At the same time, a seven-fold higher risk of acquiring myocarditis after COVID-19 compared to vaccination must be emphasized,” the organization added.
“Health care organization records have revealed that males aged 12 to 17 are most likely to develop myocarditis within three months of a COVID-19 infection at a rate of about 450 million,” said PSAAI.
Several health professionals in the US, meanwhile, have been criticizing a preprint study citing data from VAERS on the risk of 12-15 year old healthy boys experiencing cardiac adverse events, like myocarditis, following doses of Pfizer vaccine.
Oxford University Prof. Trish Greenhalgh said the VAERS database “is a passive monitoring system that invites the public to report any perceived or suspected side effects following vaccination so that potential signals of harm may be investigated further.”
“Crucially, all such reports must be validated by other active monitoring systems, as VAERS entries are very prone to reporting and recall bias,” Greenhalgh said in an article published in the BMJ — a weekly peer-reviewed medical journal published by the British Medical Association.
“Indeed, the CDC explicitly states that VAERS cannot be used in isolation to infer the existence, frequency, or rates of vaccine complications,” she added.
The article also said critics called out the preprint study for allegedly using data from an inappropriate source — which could deliver an anti-vaccine message.
“VAERS data dredging, as it is known, has been used by anti-vaccine groups in the past to produce alarmist estimates of harms from vaccines,” Greenhalgh said.
PSAAI, in its statement, also said that there have been no cases of anaphylaxis, immune thrombocytopenia, or other significant reactions reported in clinical trials.
Still, the organization warned of the possibility that such reactions may occur.
“Therefore, careful history-taking and risk assessment are a must prior to and after each vaccination.”
Managing allergic reaction
PSAAI also issued some recommendations for eligible minors before the first and second doses of mRNA vaccines.
The list of recommendations stated that the following patients may get vaccine shots but with proper guidance from qualified specialists:
- Patients who are allergic to other types of vaccines and injectable medications, food, inhalant or environmental allergens, insects, latex, and oral medication.
- Patients suffering from immunodeficiency, cancer, and autoimmune disease.
- These patients should also undergo proper evaluation and thorough discussion of vaccine risks and benefits by their doctors.
- Individuals with a history of non-vaccine-related myocarditis or pericarditis must be given a proper evaluation and thorough discussion of vaccine risks and benefits by a specialist.
The following individuals may receive doses of COVID vaccines:
- Persons with non-anaphylactic reactions to food, inhalant or environmental allergens, insects, latex, and oral medications not related to vaccines and their components.
- Patients with well-controlled asthma, allergic rhinitis, atopic dermatitis, and chronic urticaria — both those who are on medications and those who are not.
- Vaccine recipients with local and systematic reactogenic reactions and stress-related reactions including syncope after getting the first dose of vaccine can still receive the second dose of COVID vaccine.
Severe immediate allergic reactions, like anaphylaxis, and serious adverse events such as myocarditis to a previous dose of vaccine are considered contraindications to the second dose.
“A referral to a qualified specialist for evaluation is recommended,” the organization advised.
TSB
For more news about the novel coronavirus click here.
What you need to know about Coronavirus.
For more information on COVID-19, call the DOH Hotline: (02) 86517800 local 1149/1150.
The Inquirer Foundation supports our healthcare frontliners and is still accepting cash donations to be deposited at Banco de Oro (BDO) current account #007960018860 or donate through PayMaya using this link.