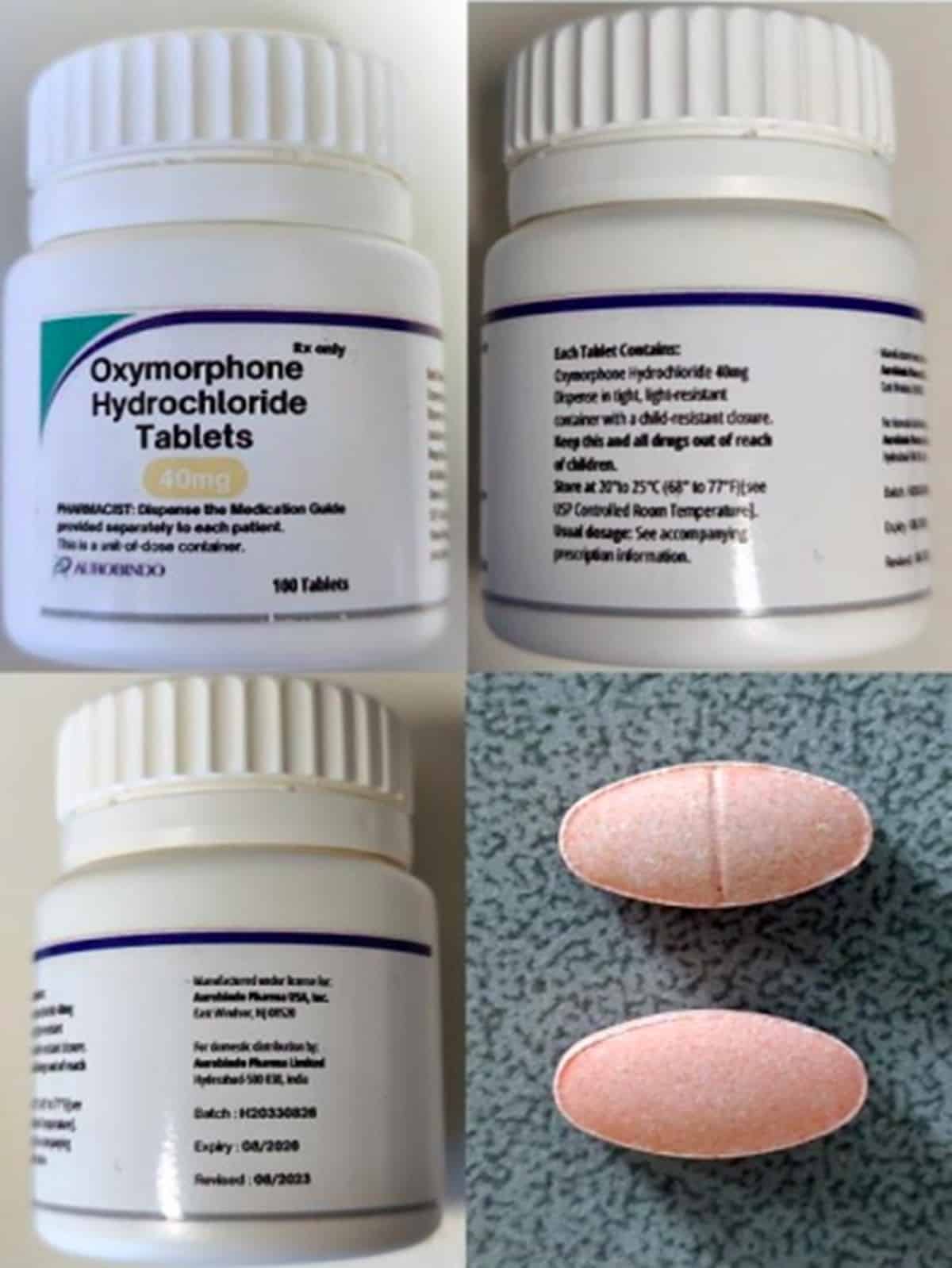
FAKE AND DEADLY The public has been warned to watch out for fake Oxymorphone Hydrochloride 40 mg tablets which contain a drug that can cause death. —Food and Drug Administration
MANILA, Philippines — The Food and Drug Administration (FDA) has warned the public to watch out for fake versions of a US-made pain reliever that is laced with an addictive illegal substance that can cause death.
Under FDA Advisory No. 2024-1325 issued on Wednesday, the FDA said fake Oxymorphone Hydrochloride 40 milligram tablets might be circulating in the country, specifically those marked Batch No. H20330826 with an expiration date of August 2026. Its stated manufacturer is a certain Aurobindo Pharma Ltd. based in India.
READ: FDA: 6 widely used OTC drugs faked, sold publicly
The fake drug product, the FDA said, contains metonitazene, “a potent psychoactive synthetic opioid drug that is not officially recognized or authorized for medicinal or therapeutic use.”
“Small doses of this substance can result in serious adverse effects such as respiratory depression, severe sedation, addiction, and an overdose which may lead to death,” it added.
Prohibited substance
Metonitazene is among the newest prohibited substances in the country, added only to the Dangerous Drugs Board’s list of illegal drugs in 2022.
Oxymorphone Hydrochloride, a drug used to treat moderate to severe pain, is three to five times more potent than morphine, a powerful opium-based pain reliever.
It is also used as a sedative before surgery, to help with anesthesia during surgery and labor, and to treat anxiety caused by some medical conditions.
The FDA noted that Oxymorphone Hydrochloride 40 mg tablet is not registered in the Philippines. “It is important to detect and remove this product from circulation to prevent harm to patients,” it said.
Its unauthorized sale or distribution may be reported to the FDA Center for Drug Regulation and Research at cdrr.od@fda.gov.ph, or by calling (02) 8809-5596.
The owner of the genuine medicine, Aurolife Pharma Llc. based in New Jersey, USA, confirmed that the product and its variable data referenced were falsified, including the packaging of the product.
To tell the real from the fake, the World Health Organization asked the public to check the following: The falsified version label does not have a barcode on the bottle and is labeled 40 mg. The genuine drug is available only in 5 mg and 10 mg. The fakes also lack embossed letters and numbers, while its labels do not bear the US National Drug Code.