FDA: 6 widely used OTC drugs faked, sold publicly
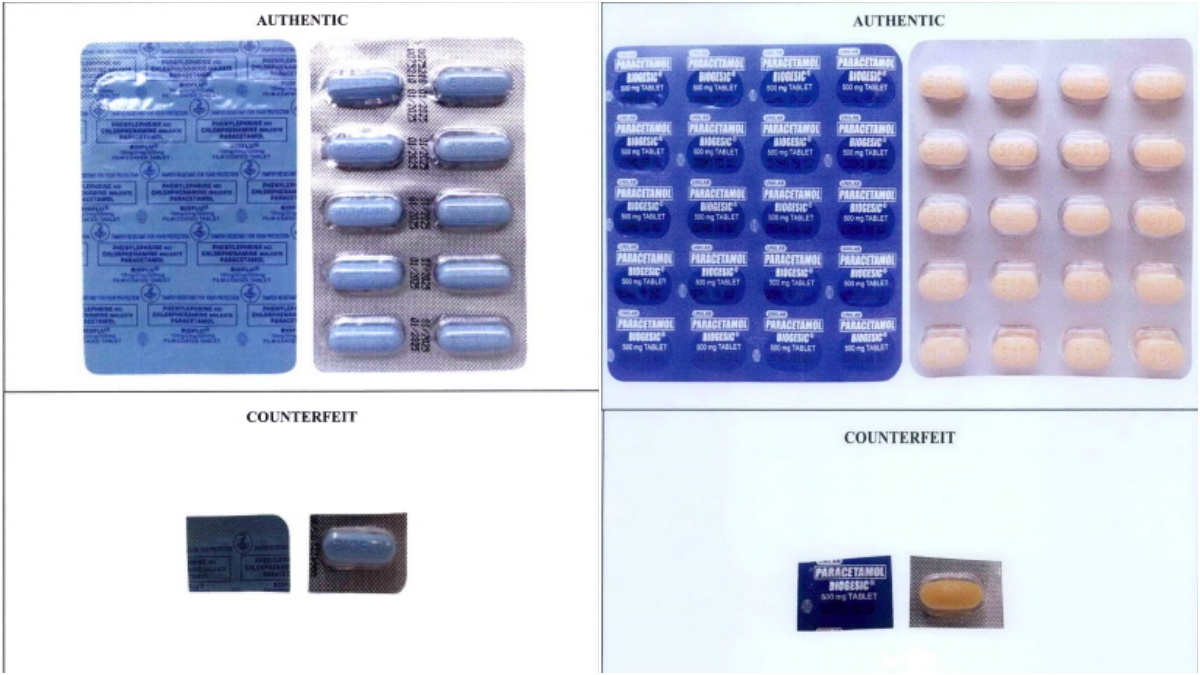
FAKE VS REAL Pharmaceutical company Unilab says that compared with the real medicines, the fake ones may be too light, too dark or unusual in color. —photos from the Food and Drug Administration
MANILA, Philippines — The Food and Drug Administration (FDA) has warned the public to exercise caution when buying over-the-counter (OTC) medication after it discovered counterfeit versions of commonly used medicines manufactured by a local pharmaceutical company.
In two advisories dated May 17 but released only recently, FDA Director General Samuel Zacate reported that they found counterfeit versions of Kremil S, Alaxan FR, Biogesic, Medicol Advance, Bioflu and Tuseran Forte, all made by Unilab.
Kremil-S (Aluminum Hydroxide + Magnesium Hydroxide + Simeticone) is used to relieve stomach pain due to hyperacidity.
On the other hand, Alaxan FR (Ibuprofen + Paracetamol), Biogesic (Paracetamol) and Medicol Advance (Ibuprofen) are taken for headaches and body pains.
Bioflu (Phenylephrine Hydrochloride + Chlorphenamine Maleate + Paracetamol) and Tuseran Forte (Dextromethorphan Hydrobromide + Phenylpropanolamine Hydrochloride + Paracetamol) are used to treat cough, colds, fever and other flu symptoms.
Article continues after this advertisementREAD: FDA warns vs increasing fake versions of popular drugs
Licensed stores only
“All health-care professionals and the general public are hereby warned as to the availability of these counterfeit drug products in the market, which pose potential danger or injury to consumers. Consumers are also reminded to purchase drug products only from FDA-licensed establishments,” the FDA said.
READ: FDA intensifies crackdown vs fake meds sold online
The sale of fake drugs is punishable under Republic Act No. 9711, or the FDA Act of 2009, and RA 8203, or the Special Law on Counterfeit Drugs. Those found in possession of counterfeit medicines may face imprisonment of not less than six months and one day.
Unilab also issued a warning on Monday against fake medicines and vitamins, saying these might contain dangerous substances like chalk, cornstarch, flour, pollen, rat poison, arsenic or cement as a replacement for the medications’ active ingredients.
“Since most fake medications try to pass [for] genuine products, patients who try to ingest them may be at risk [of] failing to get the desired effects, such as managing a symptom or treating an illness,” the company said in a post on its official Facebook page.
“[They may also] ingest toxic ingredients … which, if done [over] an extended period of time, can [result in] serious health conditions and may lead to hospitalization or death,” it added.
READ: FDA warns vs unregistered Vitabears supplements
How to spot fakes
To identify fake medical products, Unilab asked consumers to be on the alert for colors that may be too light, too dark or unusual compared with the real ones. Counterfeit drugs may also have a different size or shape, an unusual smell, or mold or dirt on them.
Other things to look out for are misspelled words, grammatical errors on the packaging or a missing expiration date and lot number. The packaging and security seal may also be dirty, tampered with, damaged or printed using substandard materials.
In an earlier interview with Unilab officials, they said law enforcement agencies found counterfeit drugs being sold in retail stores, which usually do not have a license to sell medicine from the FDA.
In 2022, the Department of the Interior and Local Government directed local governments—which are in charge of issuing business licenses to retail stores—to pass ordinances banning the sale of medications in such establishments nationwide.
This was after the FDA received a total of 185 reports in just one month about retail stores caught illegally selling medicines. Of the total, nine stores were selling fake drugs, including COVID-19 medication.