US FDA set to authorize COVID-19 vaccine boosters for immunocompromised patients
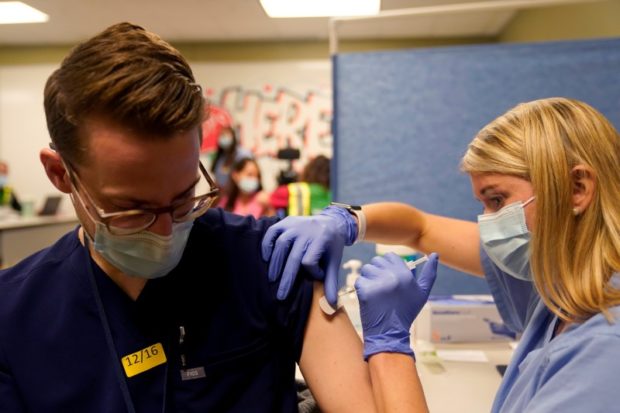
Fourth-year medical student Anna Roesler administers the Pfizer-BioNTech coronavirus disease (COVID-19) vaccine at Indiana University Health, Methodist Hospital in Indianapolis, Indiana, U.S., December 16, 2020. REUTERS FILE PHOTO
The U.S. Food and Drug Administration is expected to authorize a third booster dose of COVID-19 vaccines by Pfizer Inc and Moderna Inc for people with weakened immune systems, NBC News reported on Wednesday.
The health agency will amend the emergency use authorizations for the two vaccines as soon as Thursday to allow immunocompromised people to get an additional dose, the report said, citing people familiar with the matter.
The agency is closely monitoring data as it becomes available from studies administering an additional dose of the authorized COVID-19 vaccines to immunocompromised individuals, FDA spokesperson Abby Capobianco said in a statement.
“The FDA, along with the CDC, is evaluating potential options on this issue, and will share information in the near future,” Capobianco said.
A U.S. Centers for Disease Control and Prevention (CDC) advisory panel will meet on Friday to review data on booster doses in immune compromised individuals. While there is no vote listed on the draft agenda for the meeting, the Federal Register entry said a recommendation vote is scheduled.