Duterte: ‘I’m open in the use of Dengvaxia again’
The Daily Star/Asia News Network
MANILA, Philippines — President Rodrigo Duterte on Thursday said he is open to using Dengavaxia again amid the prevailing dengue epidemic in the country.
“I’m open in the use of Dengavxia again. Maraming patay na, it’s an epidemic,” Duterte told reporters in an interview in Malacañang.
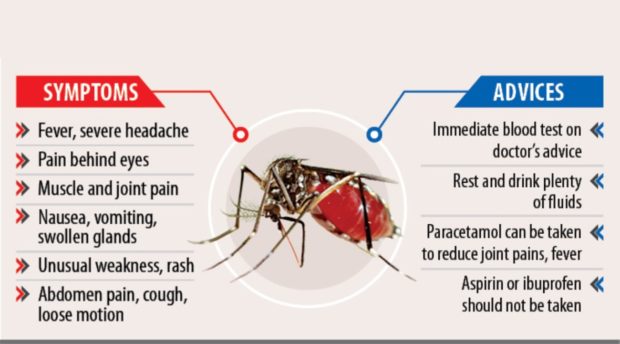
The government has declared a national dengue epidemic on Tuesday after recording an infection rate of 5,100 cases per week.
The Department of Health (DOH) has so far documented 146,062 cases of dengue, with 622 deaths as of July 20. This is double from the 73,818 cases recorded in the same period last year.
READ: DOH declares national dengue epidemic
But Duterte said he will wait for and heed the decision of health experts about the possible reuse of Dengavaxia.
Article continues after this advertisement“I want to hear the words of the experts, doctors. And we have enough bright people here to tell us. I do not need foreigners to tell me, my own Filipino scientists and doctors would tell me what to do. I will be guided by their announcements,” he said.
INQUIRER FILE PHOTO
Health Secretary Francisco Duque III said they will decide within the next two weeks whether the government will reintroduce Dengvaxia amid surging cases of the mosquito-borne disease.
READ: DOH to decide in two weeks on the use of Dengvaxia
DOH stopped the school-based dengue immunization program in 2017 after Dengvaxia manufacturer Sanofi Pasteur issued a belated communication that the vaccine may lead to more severe symptoms of dengue for those who have never been infected by the virus prior to inoculation.
Consequently, the Food and Drug Administration (FDA) permanently revoked Dengvaxia’s certificate of product registration in the country early this year even if DOH said in January 2019 that no death had been confirmed to have been directly caused by the vaccine.
Dengvaxia “is the first dengue vaccine to be licensed. It was first licensed in Mexico in December 2015 for use in individuals 9-45 years of age living in endemic areas, and is now licensed in 20 countries,” according to the World Health Organization. /kga
RELATED STORY