The story of emulsion
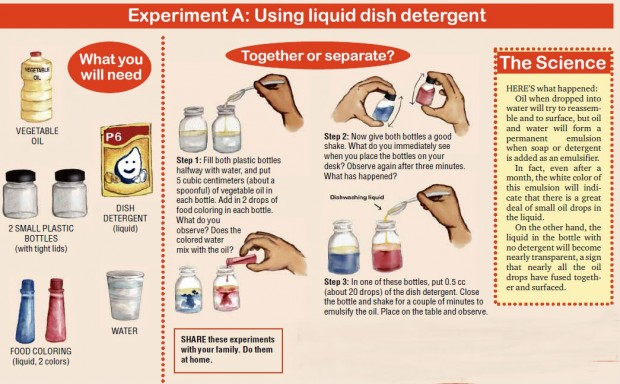
If water and oil do not mix, is there a way to combine them? The answer is yes, with some help from an emulsifying agent.
Emulsifying agents are substances that are soluble in both fat and water. They enable fat to be uniformly dispersed in water.
When stirred or shaken vigorously, the oil and water will form a temporary emulsion. Within a relatively short time, however, the two will separate into distinct layers.
A permanent emulsion can be made by adding a third substance, called an emulsifier or emulsifying agent, to the mixture.
To demonstrate the action of an emulsifier, let’s do Experiment A.
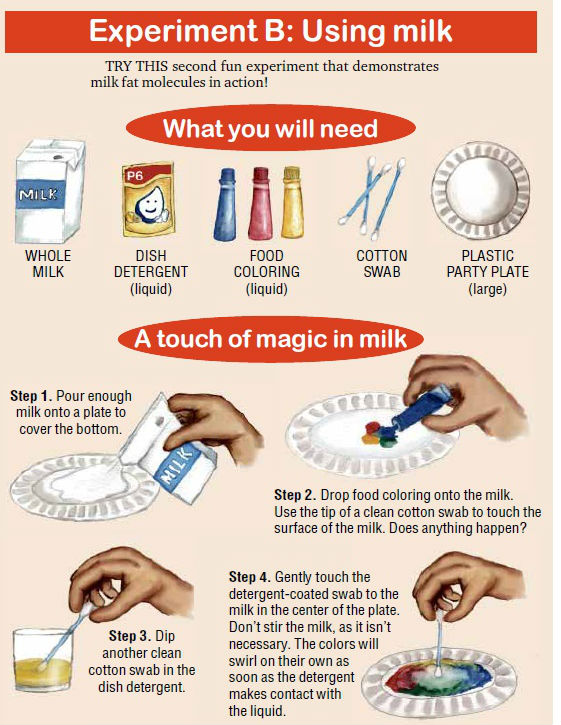
Then let’s do Experiment B using a permanent emulsion such as milk and see what happens if you add simple household ingredients to milk.
Article continues after this advertisementAbout the series
Article continues after this advertisementTHE BAYER Smiling Kiddie Einsteins series offers students, teachers and even parents hands-on and inquiry-based experiences that involve observing, hypothesizing, analyzing and testing.
Through this series of experiments related to health, nutrition and nature, Bayer and Inquirer in Education aim to deepen the interest of elementary school pupils in Science.
The materials needed for these experiments are safe and can easily be accessed from your home.
The Science
HERE’S what happened:
Milk consists of a lot of different types of molecules, including fat, protein, sugars, vitamins and minerals. It is a permanent emulsion of butterfat in water, with casein acting as the emulsifier.
When you touch a clean cotton swab to the milk, not much happens. The cotton is absorbent, so you create a current in the milk, but nothing dramatic happens.
When you introduce detergent to the milk, several things happen at once.
The detergent lowers the surface tension of the liquid so that the food coloring is free to flow throughout the milk. It reacts with the protein in the milk, altering the shape of those molecules and setting them in motion.
Eventually, equilibrium is reached, but the swirling of the colors continues for quite a while before stopping.
The reaction between the detergent and the fat forms micelles. As the micelles form, the pigments in the food coloring get pushed around.
This experiment explains how detergents lift grease off dirty dishes and clean our clothes in the laundry.
Detergents and soaps are used for cleaning because pure water cannot remove oily, organic soiling. Soap cleans by acting as an emulsifier. Soap allows oil and water to mix so that oily grime can be removed during rinsing.
Using the newspaper
“OIL AND WATER don’t mix” is an idiom in the English language. Look up what it means in the dictionary.
It used to be that two people of different races could not marry because of cultural, religious and other differences. But today mixed couples are common.
Cut out news about a very popular person who is a product of a mixed marriage; glue on a sheet of paper.