US regulators approve new tuberculosis drug
The Food and Drug Administration (FDA) said it was approving the drug, named Sirturo, as an alternative treatment for adults suffering from TB when two more powerful medications that are available, isoniazid and rifampicin, do not work.
But the regulator cautioned that the new drug should be used sparingly.
“Sirturo provides much-needed treatment for patients who have don’t have other therapeutic options available,” announced Edward Cox of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research.
“However, because the drug also carries some significant risks, doctors should make sure they use it appropriately and only in patients who don’t have other treatment options.”
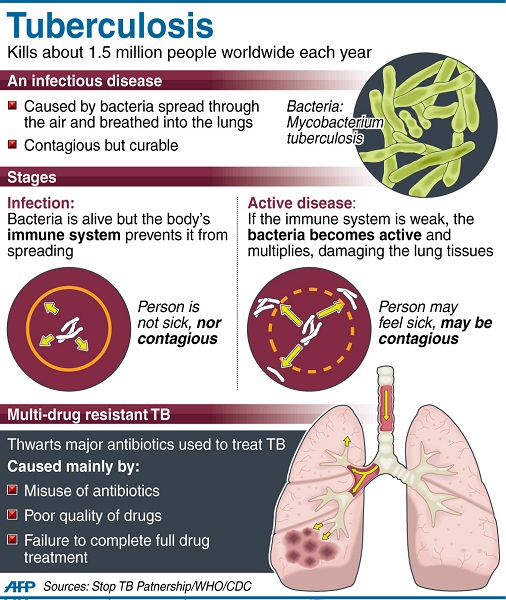
TB is an infection caused by Mycobacterium tuberculosis and is one of the world’s deadliest diseases. It is spread from person to person through the air and usually affects the lungs, but it can also affect other parts of the body such as the brain and kidneys.
Article continues after this advertisementNearly nine million people around the world had TB in 2011, including more than 400,000 suffering from a multidrug resistant form of the disease, according to the World Health Organization.
Article continues after this advertisementThe FDA said Sirturo will carry a boxed warning to alert patients and health professionals that the drug can affect the heart’s electrical activity, which could lead to an abnormal and potentially fatal heart rhythm.
The warning also noted deaths in patients treated with Sirturo and that nine patients who received Sirturo died — compared with two patients who received a placebo.
Sirturo’s manufacturer, Janssen Therapeutics, based in the US state of New Jersey, will distribute the drug from a single source and provide advisory literature to help ensure the new product is used appropriately, the FDA said.