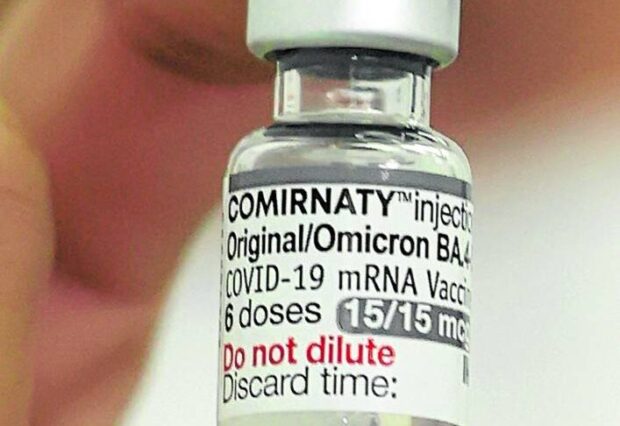
Pfizer will sell the vaccines under the brand name Comirnaty Original/Omicron BA.4-5. (Photo by NIÑO JESUS ORBETA / Philippine Daily Inquirer)
MANILA, Philippines — A doctor’s prescription is required for the purchase of bivalent COVID-19 vaccines, according to the Food and Drug Administration (FDA).
The bivalent jab Tozinameran + Famtozinameran is expected to be available in drugstores soon, after the FDA said earlier this week that it had granted its manufacturer, Pfizer, a certificate of product registration (CPR) for the commercial distribution of the vaccines.
Pharmacist Jesusa Cirunay, director of the FDA’s Center for Drug Regulation and Research, said consumers are not allowed to administer the jabs upon themselves.
“It has to come with a prescription because it’s an Rx product … and it has to be injected by a licensed [health professional],” she said at a press briefing on Friday.
The symbol Rx on a medical product indicates that it requires the prescription of a physician.
Drugstores may tap “trained immunizing pharmacists,” who are also licensed to do the vaccination, Cirunay said, adding that this will help simplify the process for people who need the bivalent jabs.
‘Proper refrigeration’
Pharmacies would also be monitored if they have proper cold storage for the vaccines.
The jabs “should be kept in proper refrigeration, 2 to 8 degrees Celsius for 10 weeks,” Cirunay said, adding that this was a specific requirement since the vaccines now have “marketing authorization.”
Under the CPR, the jabs may be sold to consumers 12 years old and above.
Pfizer will sell the vaccines under the brand name Comirnaty Original/Omicron BA.4-5 upon their shipment to the country, following an agreed price with the FDA.
The vaccine specifically targets the fast-spreading BA.4 and Ba.5 subvariants of the Omicron variant of the coronavirus.
Despite the presence of these subvariants in the country, infectious disease expert Rontgene Solante said in November last year that “We have already surpassed the worst” of their transmission.
‘Studying the price’
Pfizer had also said earlier that it is still “studying the price” of Tozinameran + Famtozinameran for the Philippine market.
Lawyer Emilio Polig Jr., director of FDA’s legal services support center, said “We first have to wait on what would be the declared price of Pfizer… It’s a drug product, so under the Price Act (Republic Act No. 7581), it should be monitored by the DOH (Department of Health).”
FDA Director General Samuel Zacate urged other pharmaceutical companies to apply for CPR for their bivalent COVID-19 vaccines.
“I’d like to call on the public to trust the vaccine [and] have themselves vaccinated because it is the only way to end the pandemic,” he said.