After US approval, DOH gears to inoculate 5-year-olds and younger
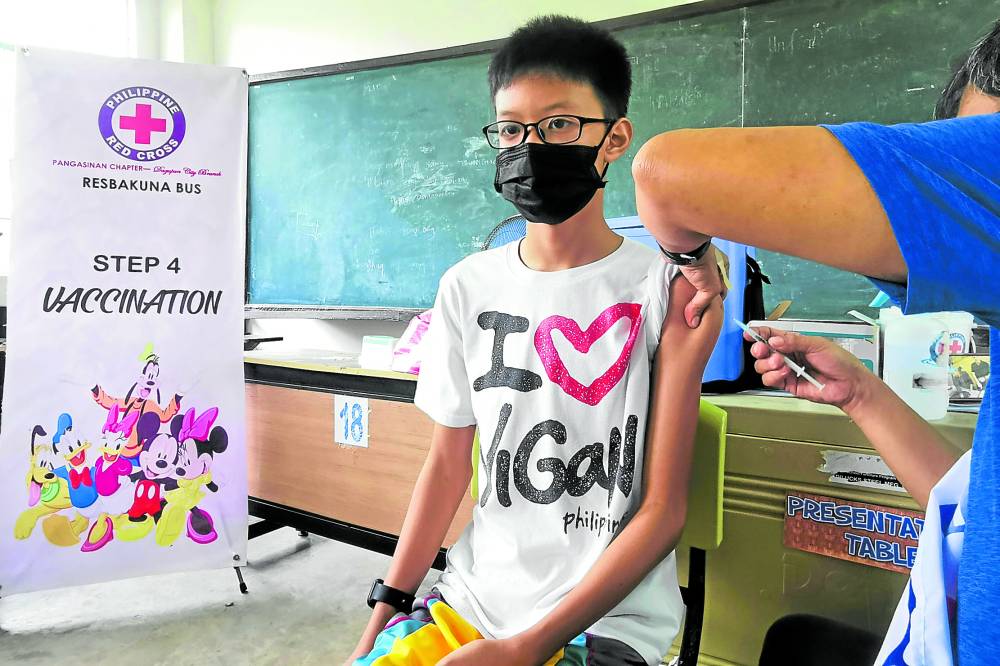
Railey Fave, 12, receives his booster shot against COVID-19 from the Philippine Red Cross and Dagupan City Health Office during the “Brigada Bakuna,” a program under the Brigada Eskwela kickoff at Dagupan City National High School.—WILLIE LOMIBAO/FILE PHOTO
After the recent approval in the United States of bivalent COVID-19 shots for children as young as six months, the Department of Health (DOH) said on Friday it is ready to vaccinate the remaining age group in the country that has not received any kind of COVID-19 vaccines.
But Undersecretary Maria Rosario Vergeire, officer in charge and spokesperson for the DOH, said they were still awaiting emergency use authorization (EUA) applications from vaccine manufacturers, such as Pfizer and Moderna.
“[We are] currently evaluating on Sinovac for below 5 years old. Let’s wait for the [results] because the EUA triggers our process for our experts to evaluate further,” she said in a press briefing.
So far, the Chinese drug company is the lone developer that has submitted its EUA application for the youngest age group to the Food and Drug Administration (FDA).
But Vergeire expects Pfizer and Moderna to “apply soon” with the FDA.
Article continues after this advertisement“As long as there’s evidence and science that say that this will be of benefit to our young children less than 5 years old, we are ready because we’ve been implementing immunization for kids for decades,” noted Vergeire.
Article continues after this advertisementBivalent vaccines are the reformulated doses designed to target the new COVID-19 strains as well as the original variant.
Meanwhile, the DOH reported that 33,239 children aged 5 to 11 were vaccinated in the three-day “Bakunahang Bayan” that closed on Wednesday. This makes 49.3 percent of the target 5 to 11 years old now vaccinated with primary doses, according to Vergeire.
Last jab days for the year
The last round of special vaccination days for this year focused on vaccinating the youngest eligible population for COVID-19 jabs.
The US health regulator announced on Dec. 8 that it had authorized COVID-19 shots from Moderna and Pfizer and its partner BioNTech for children as young as six months of age.
The amended authorization on Thursday from the US FDA allows use of Moderna’s bivalent shot as a booster in children six months through five years of age, two months after their initial vaccination.
Pfizer/BioNTech’s updated shot can now be given as a third dose to those aged six months through four years, who have not completed their primary vaccination series or are yet to receive the third dose.
Children who have completed their initial three-dose vaccination with Pfizer’s original shot are not yet eligible to receive the bivalent booster, the agency said.
The regulator added that data supporting use of Pfizer/BioNTech’s bivalent shot as a booster in this age group is expected in January.
—WITH A REPORT FROM REUTERS
RELATED STORY:
For more news about the novel coronavirus click here.
What you need to know about Coronavirus.
For more information on COVID-19, call the DOH Hotline: (02) 86517800 local 1149/1150.
The Inquirer Foundation supports our healthcare frontliners and is still accepting cash donations to be deposited at Banco de Oro (BDO) current account #007960018860 or donate through PayMaya using this link.