Artificial heart maker CARMAT gets regulatory approvals to resume sales
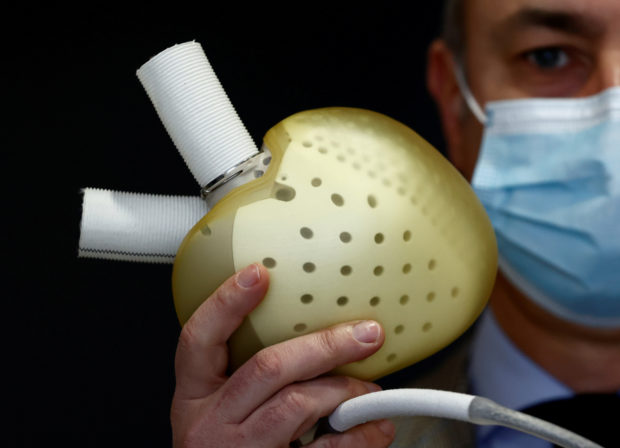
Chief Executive Officer of French artificial heart manufacturer Carmat, Stephane Piat, poses holding an artificial heart during an interview with Reuters in Velizy, near Paris, January 11, 2021. REUTERS FILE PHOTO
PARIS — French medical devices company CARMAT said it had received the necessary regulatory approvals to resume commercial implants of its Aeson artificial hearts product and would resume sales soon.
“We will be resuming implants very soon and moving forward at a measured pace as we continue building our inventory of implantable prostheses, and ensure all patients are suitably monitored,” Chief Executive Stéphane Piat said in a statement.
RELATED STORIES
French firm announces first implant of its artificial heart in a woman
79-year-old with artificial heart reaches 1,000-day milestone