New clinical trial shows ivermectin ineffective vs COVID
INQUIRER PHOTO. GRIG MONTEGRANDE
MANILA, Philippines—The anti-parasitic drug ivermectin, which was considered by some as an effective alternative treatment for SARS-CoV-2 despite the lack of evidence, was found to have no sign of any clinical benefit against COVID-19, according to results of a new clinical trial.
Ivermectin has attracted much attention and gained popularity amid the pandemic as an effective alternative COVID treatment.
The drug, which has been widely used to treat parasitic infections in animals, was among the thousands of drugs that were experimented with and tested by researchers early in the pandemic, in hopes to find treatment for the disease.
In the Philippines, during the past two years, groups of medical experts and advocates of ivermectin as an alternative treatment for COVID have been exchanging arguments on whether or not the drug should be used in the country for COVID.
The latest clinical trial published on Wednesday (March 30) in The New England Journal of Medicine backed warnings by medical experts against the use of ivermectin for the prevention and treatment of COVID-19.
Article continues after this advertisementThe study found evidence disproving claims—that are too good to be true—about the benefits of the drugs.
Article continues after this advertisement‘No sign of any benefit’
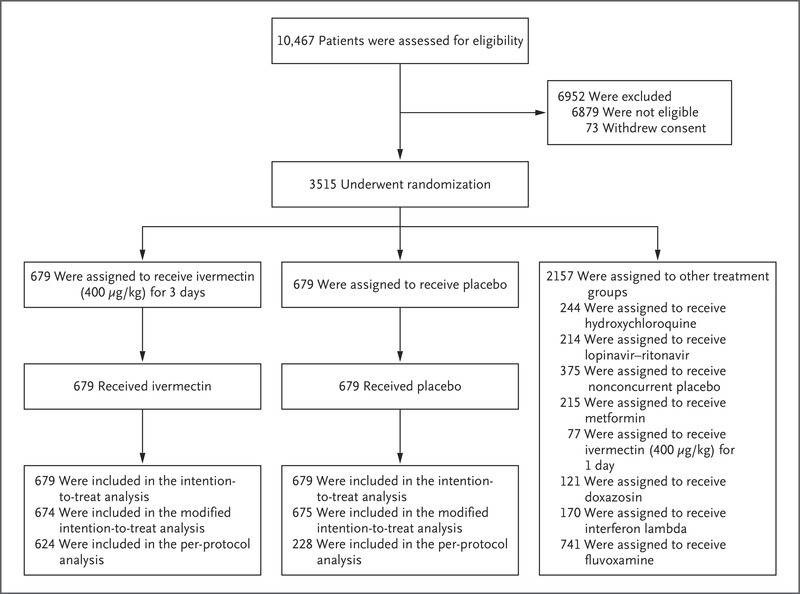
The recently released study, a large clinical trial known as TOGETHER conducted by a group of researchers between March and August 2021, tested the efficacy of ivermectin in preventing hospitalization or extended observation in an emergency setting among volunteer patients from Brazil.
The patients, who were scattered in 12 public health clinics, had had symptoms of COVID-19 for up to seven days and had at least one risk factor for disease progression.
GRAPHIC: TOGETHER team
The researchers conducted a double-blind, randomized study, which means that both patients and medical professionals, who participated in the study, were not informed beforehand whether they will be given ivermectin or a placebo.
Out of the total 3,515 patients included in the study, 679 received ivermectin—400 milligrams per kilogram of body weight—679 were given a placebo, while 2,157 received another intervention over the course of three days.
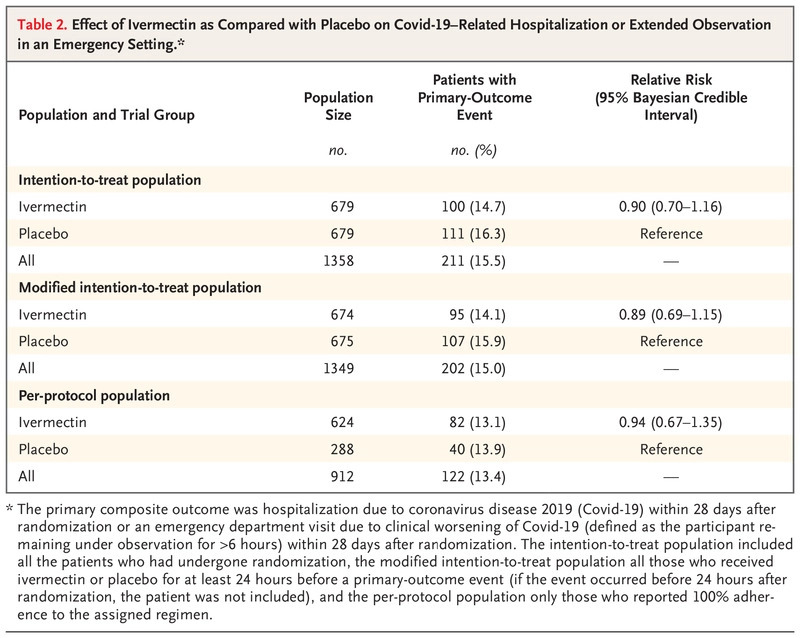
Researchers found that 211 patients had “primary-outcome events“—100 patients (14.7%) in the ivermectin group and 111 (16.3%) in the placebo group—171 (81%) of these primary-outcome events were hospital admissions.
“There were no significant effects of ivermectin use on secondary outcomes or adverse events,“ the researchers noted.
“In this randomized trial, the administration of ivermectin did not result in a lower incidence of medical admission to a hospital or prolonged emergency department observation for COVID-19 among outpatients at high risk for serious illness,“ the study concluded.
The study, as researchers stated, effectively ruled out the use of ivermectin as an alternative treatment for COVID-19.
GRAPHIC: TOGETHER team
A summary of the study’s results was previously outlined during an online presentation hosted by the National Institute of Health in August last year.
“There’s really no sign of any benefit,” Dr. David Boulware, an infectious-disease expert at the University of Minnesota said during the online presentation.
“Now that people can dive into the details and the data, hopefully, that will steer the majority of doctors away from ivermectin towards other therapies,” he added.
A more consistent outcome
Researchers in the study noted the “inconsistent“ evidence supporting the role of ivermectin in the treatment of COVID-19.
There have been three meta-analyses of clinical trials which strongly indicated the treatment benefit of ivermectin, while others have concluded that there was no benefit against COVID-19.
Over a dozen ivermectin clinical trials have been carried out and launched over the past years. At least 23 of those were reviewed in 2020 by Andrew Hill, a virologist at the University of Liverpool in England, who concluded the impact of the anti-parasitic drug in significantly reducing the risk of deaths due to COVID-19.
However, several reports showed that many of the studies reviewed and analyzed by Hill were flawed. One study, in particular, gained attention after scientists found duplicates, inconsistencies, and other causes of concern in the study’s data.
“I was shocked, as everyone in the scientific community probably were,” Eduardo López-Medina, a pediatrician at the Centre for the Study of Paediatric Infections in Colombia—who was not involved in the study—said in an article published in Nature, a peer-reviewed science journal.
“It was one of the first papers that led everyone to get into the idea ivermectin worked in a clinical trial setting,” López-Medina added.
A health worker shows a bottle of ivermectin, which some countries use to treat COVID-19. —AFP
The study was removed from a preprint platform amid investigations, while Hill retracted his original review and published another one earlier this year. In his new study, Hill and his colleagues relied on studies that were least likely to be biased and found that ivermectin has no benefit against COVID-19.
“Although the number of included trials involving outpatients varies among the meta-analyses, the overall number of events that occurred in our trial is larger than the number of all the combined events in these meta-analyses,” researchers of the TOGETHER clinical trial said.
“The results of this trial will, therefore, reduce the effect size of the meta-analyses that have indicated any benefits,” the team continued.
“A large collaboration of clinical trialists working on ivermectin treatment for COVID-19 has conducted a meta-analysis of trials and has concluded that ivermectin did not offer a treatment benefit when trials that were considered to be of moderate or better quality were examined,” they added.
The TOGETHER team also emphasized that its findings were consistent with the World Health Organization, which has previously concluded that ivermectin did not offer a treatment benefit when previous trials that were considered to be of moderate or better quality were examined.
READ: WHO: No ‘strong enough’ data to advocate Ivermectin use vs COVID-19
Conflicting views in PH
In the Philippines, the Department of Health (DOH) has repeatedly insisted and reminded the public that the anti-parasitic drug has not been proven to be effective against COVID-19.
READ: DOH warns vs use of anti-parasitic drug Ivermectin for COVID-19
READ: Ivermectin not effective in treating COVID-19, says DOH citing latest study
“Based on the current evidence from randomized controlled trials we do not recommend the use of ivermectin for the treatment of COVID-19. It has not proven to significantly reduce mortality, nor to improve other clinical outcomes,” Health Undersecretary Ma. Rosario Vergeire said in September last year after the British Ivermectin Research Development (BIRD) sent a letter asking the Philippine government to adopt the use of ivermectin for the early treatment of COVID-19.
“We must always put the safety of Filipinos first before showing the use of medicines in the country. So rest assured that the DOH and FDA (Food and Drug Administration) are at the forefront of ensuring COVID-19 drugs and medicines are safe, effective, accessible, and affordable for use,” Vergeire said.
READ: DOH: Ivermectin still not for COVID-19 treatment
Still, the FDA has so far granted six hospitals in the country a compassionate special permit (CSP) for the use of human-grade ivermectin to treat COVID-19.
READ: FDA: 6 hospitals now have special permit to use ivermectin
The FDA, however, stressed that ivermectin can only be prescribed by doctors in those hospitals that have managed to secure CSPs.
A Quezon City resident shows ivermectin capsules she received from Representatives Mike Defensor and Rodante Marcoleta last year. GRIG C. MONTEGRANDE
While doctors in the hospitals are allowed to prescribe the drug, consent from patients is still required, and they should also be informed that the drug is still an investigational product and is used only for compassionate use.
READ: Doctors warned: Don’t prescribe ivermectin outside permitted hospitals or face prosecution
The recruitment of participants in the country for the clinical trials on the use of ivermectin as COVID-19 treatment began in November, according to the Department of Science and Technology (DOST).
READ: Recruitment of participants for ivermectin trials starts in November — DOST exec
READ: PH’s clinical trial for ivermectin as COVID-19 treatment to start on Sept. 15
READ: DOST insists on conducting P22-M ivermectin clinical trial
Despite warnings from the WHO, DOH, and many more groups of medical and health experts in the country, two lawmakers last year made headlines for distributing ivermectin in Quezon City.
READ: WATCH: Defensor, Marcoleta lead ivermectin distribution in Quezon City
READ: Defensor: Who’s responsible if Ivermectin fails? ‘User’s consent will be asked’
Aside from WHO and DOH, the Philippine Society for Microbiology and Infectious Disease (PSMID) has also said that it does not recommend the use of Ivermectin for treating COVID-19.
Statement on the Use of Ivermectin As Treatment for COVID-19
Experts from the US Food and Drug Administration (FDA) and the National Institutes of Health (NIH) echoed the same sentiments against the use of the drug for the prevention and treatment of COVID-19.
TSB
For more news about the novel coronavirus click here.
What you need to know about Coronavirus.
For more information on COVID-19, call the DOH Hotline: (02) 86517800 local 1149/1150.
The Inquirer Foundation supports our healthcare frontliners and is still accepting cash donations to be deposited at Banco de Oro (BDO) current account #007960018860 or donate through PayMaya using this link.