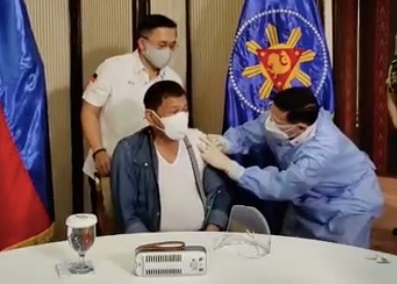
Health Secretary Francisco Duque III administers Sinopharm’s COVID-19 vaccine to President Rodrigo Duterte on Monday, May 3, 2021, in Malacañang. (Screenshot from Sen. Bong Go’s Facebook livestream)
MANILA, Philippines — President Rodrigo Duterte will still use the COVID-19 vaccine of Chinese drugmaker Sinopharm for his second dose even though he asked China to recall its 1,000-dose donation, Malacañang said Thursday.
Presidential spokesperson Harry Roque said Duterte’s second dose will not be included in the vaccines that will be sent back to China.
“Siyempre hindi ibabalik ‘yung pang-second dose ni Presidente para matapos niya ang second dose niya,” he said in a Palace briefing.
(His second dose will not be among those that will be returned, so he could finish the [vaccination].)
Roque earlier claimed that only Duterte has been inoculated with the Sinopharm vaccine but Presidential chief legal counsel Salvador Panelo earlier revealed that he also got vaccinated with the same vaccine.
In a text message to INQUIRER.net, Roque said the government will also reserve a Sinopharm vaccine for Panelo’s second dose.
In an earlier speech, Duterte said he told the Chinese ambassador to the Philippines Huang Xilian to “withdraw” the 1,000 Sinopharm vaccines the Chinese government donated.
Roque said this move will be done so that there will no longer be criticisms in the use of Sinopharm vaccines since it has not yet secured an emergency use authorization in the country, only a compassionate use permit for the Presidential Security Group.
“Sabi ni Presidente, para mawala na ‘yung ganyang kritisismo, at habang siya pa lang ang gumagamit sa 1,000 na pagbakuna, siyempre mas mabuting ibalik na muna sa Tsina,” he said.
(The President said that it’s best to return the Sinopharm vaccines to China to stop the criticisms.)
Roque, however, said that Malacañang is confident that Sinopharm, which has yet to file an official application for an EUA, will be given the EUA since it is being used by 25 other countries.
“It’s a matter of Sinopharm appointing a representative to submit ‘yung mga papeles sa FDA (Food and Drug Administration) dahil hindi naman pwedeng walang representative dito sa Pilipinas,” he said.
However, while the vaccine has no EUA yet, it will no longer be used in the country, he added.