Defensor, Marcoleta to distribute Ivermectin ‘to those in dire need of drug’
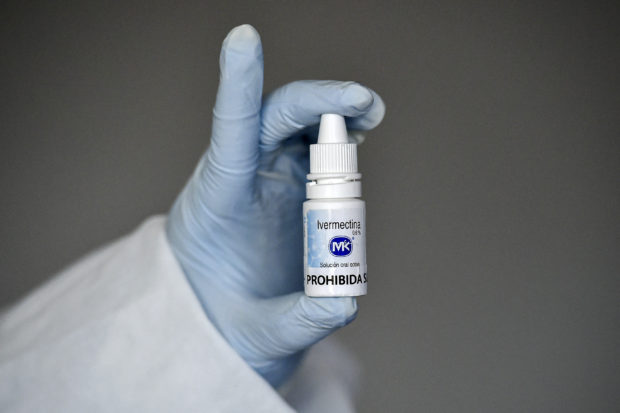
TO USE OR NOT TO USE A number of physicians swear by the effectiveness of ivermectin, an anti-parasitic drug commercially available only for veterinary use, as a treatment or preventive medicine against COVID-19. But the Philippines’ Food and Drug Administration maintains there are procedures to be followed, including clinical trials, before the drug could be allowed for distribution. —AFP FILE
MANILA, Philippines — Deputy Speaker Rodante Marcoleta and Anakalusugan Partylist Rep. Mike Defensor have reached “a critical decision” to give away compounded Ivermectin “to those in dire need of this drug” even if government regulators have yet to allow human use of the anti-parasitic medication.
The two lawmakers have named the event “Ivermectin Pan-three” wherein each beneficiary will get a minimum of three capsules or tablets of the medicine for free. The event will be formally launched on Thursday, April 29, in Matandang (Old) Balara in Quezon City.
“After the inquiries conducted by two committees of the House of Representatives where both foreign and local experts were engaged on the potential use of Ivermectin, we have decisively come to a critical decision to distribute Ivermectin to those who are in dire need of this drug,” they said in a statement.
After the kick-off event on Thursday, Marcoleta and Defensor said distribution of Ivermectin will continue to other barangays in Quezon City.
“This grave public health emergency caused by the pandemic is technically a war that needs to be decisively confronted. In war, people protect themselves with anything in order to survive. We need to cross the line and break the glass ceilings if we must, one way or the other,” the lawmakers said.
“We cannot, in good conscience, sit idly by at the excuse of inflexible bureaucracy to deny our people—especially the underprivileged—their pharmaceutically- assisted moments as they struggle to breathe their last,” they added.
While there is currently no registered Ivermectin drug in the country for human use, there are still ways that patients can legally acquire this.
Food and Drug Administration (FDA) Director-General Eric Domingo told INQUIRER.net that Ivermectin for human use can be acquired two ways: (1) through licensed compounding laboratories and pharmacies, with doctor’s prescription; and (2) through doctors and hospitals that have already secured compassionate special permit (CSP).
Marcoleta said the Ivermectin they will distribute will come from a licensed compounding lab, Lifecore Bio-Integrative, Inc, and doctors’ prescriptions will be given to beneficiaries.
“Forms will be filled out by those who wish to avail said drug, with a waiver, and doctors’ prescriptions will be accordingly issued,” Marcoleta told INQUIRER.net.
Meanwhile, Defensor said there will also be doctors present during the distribution for the beneficiaries’ prescriptions.
“Every patient will have a prescription. The doctors who are part of the Concerned Doctors and Citizens-ph (CDC-PH) will assist us on this,” Defensor told INQUIRER.net.
Asked about the legality of the process to be implemented by the two lawmakers, FDA’s Domingo said: “As long as they have doctors who will check the patients and prescribe meds that will be dispensed properly.”
No strong data
Back in March, the World Health Organization (WHO) said it is not advocating the use of Ivermectin for the treatment or prevention of COVID-19, citing a lack of “strong data” to support it.
WHO Representative to the Philippines Rabindra Abeyasinghe said prescribing Ivermectin without statistically significant evidence of its efficacy against COVID-19 is “harmful” since it would give “false confidence” to the public.
Asked who would take responsibility should there be an adverse effect on their Ivermectin beneficiaries, Defensor simply responded with another question without giving a categorical answer.
“With that question, let me reply with another question. Who shoulders the responsibility for the adverse effects of remdisivir, tocilizumab, and other medications, including vaccines, when there is an adverse effect?” Defensor said.
“There are so many recorded adverse effects for the respective drugs/vaccines they used for protocol compared to the 3.5 billion dosages in the span of 40 years that Ivermectin has been administered,” he added.
President Rodrigo Duterte has ordered the conduct of clinical trials for Ivermectin as COVID-19 treatment in the country to see its efficacy against the respiratory ailment caused by the new coronavirus SARS-CoV-2.
KGA
For more news about the novel coronavirus click here.
What you need to know about Coronavirus.
For more information on COVID-19, call the DOH Hotline: (02) 86517800 local 1149/1150.
The Inquirer Foundation supports our healthcare frontliners and is still accepting cash donations to be deposited at Banco de Oro (BDO) current account #007960018860 or donate through PayMaya using this link.