Gov’t approves cheaper, faster, safer saliva test for COVID-19
MANILA, Philippines — A laboratory experts‘ panel of the government has agreed to allow the less expensive saliva test for the new coronavirus, but this would be conducted initially only by the Philippine Red Cross (PRC), Health Undersecretary Maria Rosario Vergeire said on Saturday.
Vergeire said the PRC presented the results of its study of saliva testing for COVID-19 last week.
“It was agreed by the laboratory experts panel that this could be allowed,” Vergeire said at the Laging Handa briefing.
The experts panel’s recommendation was submitted to Health Secretary Francisco Duque III.
Vergeire said the PRC was then informed that it could use the test as an alternative to the real-time reverse transcription-polymerase chain reaction (RT-PCR) test, which the Department of Health considers the gold standard for detecting SARS-CoV-2, the virus that causes COVID-19, a severe respiratory disease.
The panel also said PRC laboratories should be used for this test for now because the results of the validation test by the Research Institute for Tropical Medicine is still being awaited.
The saliva test is the cheaper alternative to the RT-PCR swab tests, which the government requires of all passengers arriving in the country.
The saliva tests, which could also be used for nontravelers, reportedly cost P1,500 to P2,000, compared to P3,800 to P5,000 charged for the RT-PCR tests.

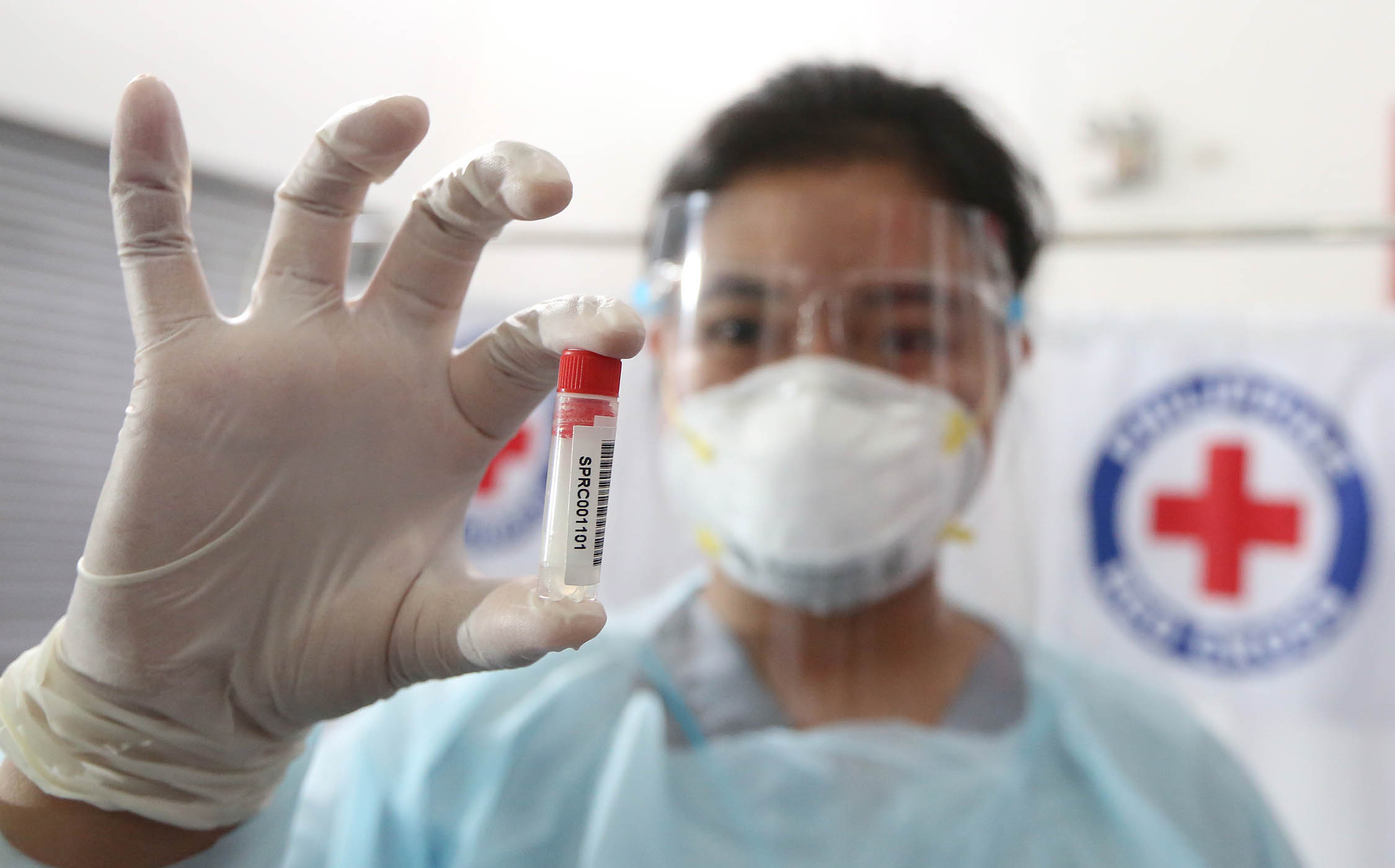
SECURE AND SPEEDY Gene Patrice Alfaro, a Philippine Red Cross (PRC) medical technician, shows a vial of saliva to be tested for SARS-CoV-2, the virus that causes COVID-19, during the pilot testing of the alternative to the more expensive real-time reverse transcription polymerase chain reaction test in this file photo taken on Jan. 12 at the PRC headquarters in Mandaluyong City. Sen. Richard Gordon, who is also PRC chair, shows how a patient provides saliva for the test that promises to be speedier and more secure as health workers will have no direct contact with the patient to collect sample specimen. —PHOTOS BY NIÑO JESUS ORBETA
More accurate
The government now requires passengers coming from countries with the UK COVID-19 variant to be tested a second time five days after the first test.
These passengers would be tested upon arrival and quarantined until the results of the second test are released.
Vergeire explained that the second test was necessary because people exposed to the coronavirus do not immediately show symptoms or test positive.
The appropriate time to get accurate test results is on the fifth to the seventh day when a person’s virus load is higher, she said.
This was also the reason some passengers test negative on arrival, but are then found positive for COVID-19 when they reach their local destinations, she said.
“We want to prevent this … which is why we put in place that measure so that we can be more accurate in our testing implementation,” she added.
Also considered a faster alternative test, the saliva test is deemed “less invasive” than the RT-PCR test, which requires collecting samples using nasopharyngeal or oropharyngeal swabs.
The PRC study found the saliva test to be 98.11-percent accurate.
Eric Domingo, director general of the Food and Drug Administration, said a saliva test is considered safer than a swab test as the sample is self-collected and would not require direct contact with a health personnel.
It was in August last year when a clinical trial for a saliva test was conducted in Israel using an artificial intelligence-based device, with the aim of determining in less than a second whether a person is infected with the coronavirus.
Increased capacity
Patients rinse their mouth with a saline wash and spit into a vial, which will be examined by a small spectral device that shines light on the specimen and analyzes the reaction to see if it is consistent with COVID-19.
The United States Food and Drug Administration issued in August 2020 an emergency authorization to Yale School of Public Health to use its SalivaDirect test kit.
The kit does not require a separate nucleic acid extraction step that was prone to shortages, which increases the capacity for testing.
A saliva-based test was also approved in Singapore last December, but patients must draw saliva from deep within the throat. Hong Kong and Taiwan are among the other places in the world that have been using this method of getting saliva samples to test for coronavirus. —WITH REPORTS FROM INQUIRER RESEARCH