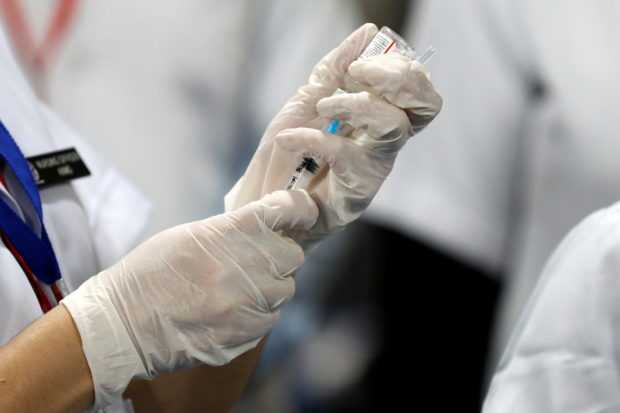
FILE PHOTO: A healthcare worker fills a syringe with a dose of Bharat Biotech’s Covid-19 vaccine called Covaxin, during the coronavirus disease vaccination campaign at All India Institute of Medical Sciences (AIIMS) hospital in New Delhi, India, January 16, 2021. REUTERS/Adnan Abidi
MANILA, Philippines — India-based firm Bharat Biotech has applied for emergency use authorization (EUA) for its Covid-19 vaccine Covaxin, the Food and Drug Administration (FDA) said Thursday.
“They submitted an application this morning and pre-evaluation is going on,” FDA Director-General Eric Domingo told INQUIRER.net in a text message.
Securing an EUA will allow a new vaccine to be administered in the country.
Other companies seeking EUA approval before the Philippine regulators are British firm AstraZeneca, Russian company Gamaleya Research Institute and Chinese drug maker Sinovac Biotech.
So far, only US pharmaceutical giant Pfizer has secured the FDA clearance for its vaccine’s use in the Philippines.