Explainer: Facts about 7 COVID-19 vaccines Philippines may get
MANILA, Philippines — As the country pursues avenues to secure COVID-19 vaccines, prices of available and soon-to-be-available COVID-19 vaccines started to evoke curiosity.
The senators, for one, have begun analyzing the price tags of the seven vaccine brands that the Philippines might obtain for its planned inoculation program.
Data released by Senate committee on finance chair Sonny Angara detailed the prices of the different vaccines for two doses per person. The total price of each vaccine is inclusive of the value-added tax (VAT) and the cost of inflation.
It also stated how many Filipinos could be inoculated based on the vaccine’s price and the P82.5 billion budget allotted by the Philippine government for acquiring COVID-19 vaccines next year.
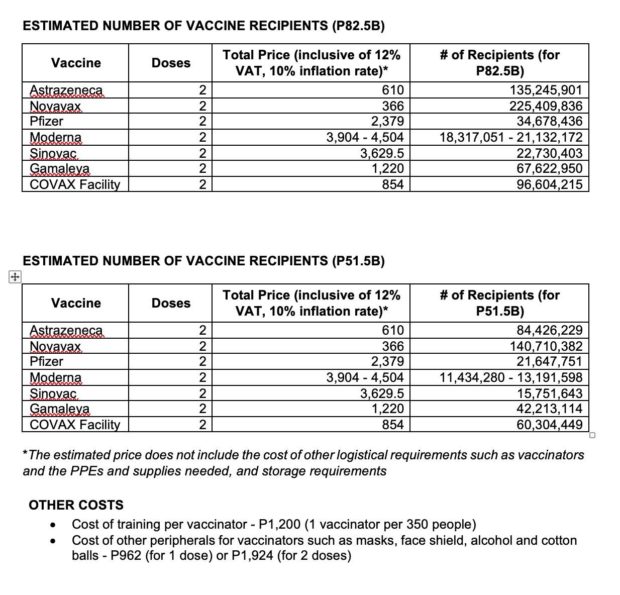
Data from Senator Sonny Angara’s Office
Moderna
According to Angara’s data, Moderna’s vaccine will cost around P3,904 to P4,504 for two doses per person. This is the most expensive among the list of vaccines based on the senator’s information.
Approximately 18,317,051 to 21,132,172 Filipinos may receive this vaccine under the earmarked P82.5 billion budget for COVID-19 vaccines, Angara said.
Article continues after this advertisementAbout Moderna: Moderna is a Massachusetts-based biotechnology company that focuses on drug discovery and development. It also makes vaccines based on mRNA (messenger RNA).
Article continues after this advertisementHow their vaccine works: The Centers for Disease Control and Prevention (CDC) website said the vaccine, also known as “mRNA-1273,” basically uses mRNA, a genetic molecule that carries the instructions for making proteins in our body. This vaccine requires two shots that will be given 28 days apart.
The mRNA in the vaccine will instruct cells to make a harmless spike protein – which can also be seen on the surface of SARS-CoV-2, the new variant of coronavirus that causes respiratory disease COVID-19, said the CDC website.
According to the CDC, the protruding spikes and spike fragments in the cell will then be recognized by the immune system and cause an immune response that produces antibodies.
These antibodies stop the coronavirus from attacking other cells by attaching to the virus’s spikes. The antibodies can also mark the virus so other cells can detect and destroy it.
Moderna’s vaccine needs to be stored at -20C.
Status development: Moderna announced last month that it has finished the first interim analysis of the Phase 3 study for mRNA-1273. According to the company, the study showed that its vaccine has an efficacy rate of 94.5 percent.
Issues and concerns: Based on a study published in The New England Journal of Medicine, Moderna’s vaccine can protect for at least three months.
But according to a report by the New York Times, since the vaccine is new, researchers still have no idea how long the vaccine’s protection will last.
The report added that the number of antibodies and T-cells might reduce months after the vaccination. However, memory B-cells and memory-T cells might retain the information about the coronavirus even after years or decades.
Roll-out: Moderna applied for emergency use authorization to the U.S. Food and Drug Administration (USFDA) on November 30. USFDA is set to decide on its application on December 17. If approved, Moderna can start releasing its vaccines in the US before the end of the month.
Sinovac
The second-most-expensive vaccine on the data is Sinovac with a price tag of P 3,629.50 for two doses per person. Some 22,730,403 Filipinos may benefit from the vaccine if acquired by the government, according to Angara’s information.
About Sinovac: Sinovac is a Beijing-based biopharmaceutical company founded in 2001. Their focus is on the research, development, and manufacture of vaccines against infectious diseases such as H1N1 influenza and COVID-19.
How their vaccine works: Unlike Moderna, which uses RNA in its vaccines, Sinovac’s CoronaVac relies on inactivated pathogens that are grown and killed in laboratories.
Similar to the inactivated vaccines used for polio, this vaccine uses the killed viruses to produce antigens which signal the immune system to attack the virus causing COVID-19.
CoronaVac can be stored in a standard refrigerator at 2-8 degrees Celsius.
Status development: In October, Sinovac submitted its application to the Food and Drug Administration (FDA) of the Philippines to conduct Phase 3 clinical trials for CoronaVac in the country.
READ: After passing PH vaccine panel evaluation, Sinovac seeks FDA OK
COVID-19 policy chief implementer and vaccine czar Carlito Galvez, Jr. earlier said that the vaccine may be the first to be rolled out in the country, probably by the first quarter of 2021.
Sinovac has yet to report the efficacy rate of CoronaVac.
Issues and concerns: Sinovac has been recently reported to be involved in a bribery scandal. However, the company cleared out its CEO, Yin Weidong, of the accusations.
Roll-out: According to Galvez, they are already finalizing negotiations with Sinovac. He said the pharmaceutical firm vowed to supply vaccines in the country by April 2021. However, Galvez negotiated to get it by March.
Pfizer-BioNTech
Pfizer and its German partner BioNTech’s vaccine costs P2,379 for two doses per person. Data showed that roughly 34,678,436 Filipinos may get the shots based on the government’s allocated fund for vaccine procurement next year.
About Pfizer and BioNTech: US-based multinational pharmaceutical corporation, Pfizer, is also known as one of the world’s largest pharmaceutical companies.
On the other hand, BioNTech SE is a German biotechnology company that focuses on individualized immunotherapies.
How their vaccine works: Pfizer-BioNTech’s vaccine is similar to Moderna’s mRNA-1273 in the sense that it uses mRNA to eliminate the new coronavirus SARS-CoV-2.
Pfizer-BioNTech’s vaccine requires storage with a temperature of -70 degrees Celsius.
Status development: Pfizer and BioNTech concluded the Phase 3 study for their vaccine on November 18. Meanwhile, the USFDA on December 12 granted an emergency use authorization for the vaccine, and on December 14 started its use in New York City.
The United Kingdom, the first country in the world to conduct mass inoculation against COVID-19 starting December 8, likewise used the vaccine developed by Pfizer and BioNTech.
On Monday, Singapore Prime Minister Lee Hsien Long announced the approval of the Pfizer-BioNTech vaccine for his country’s mass vaccination program that will likely begin by end-December. Later, Jordan announced it had approved emergency use of the Pfizer-BioNTech coronavirus vaccine.
Pfizer’s vaccine showed a 95 percent efficacy rate during its study.
Issues and concerns: U.K.’s Medicines and Healthcare Products Regulatory Agency (MHRA) on December 10 issued an anaphylaxis warning on the Pfizer-BioNTech vaccine after reports of three cases of adverse reactions.
According to MHRA, there were two reports of anaphylaxis and one possible allergic reaction after administering the vaccines.
“Any person with a history of anaphylaxis to a vaccine, medicine or food should not receive the Pfizer BioNTech vaccine,” MHRA Chief Executive June Raine said in a statement.
“Most people will not get anaphylaxis and the benefits in protecting people against COVID-19 outweigh the risks… You can be completely confident that this vaccine has met the MHRA’s robust standards of safety, quality and effectiveness,” Raine assured.
Roll-out: Pfizer-BioNTech began its first shipment of the vaccines to several states in America on December 13.
Gamaleya
Named after the first Soviet space satellite, Gamaleya Research Institutes’s Sputnik V vaccine is worth P 1,220 for two doses per person. According to the data provided by Angara, with its price, the vaccine may be administered to 67,622,950 Filipinos using the P82.5 billion budget.
About Gamaleya: The Gamelaya Research Institute of Epidemiology and Microbiology is a Russian medical-research institute founded in 1891.
According to Gamelaya, it “runs one of the unique “virus libraries” in the world and has its own vaccine production facility.”
Aside from their research for the COVID-19 vaccine, they are also working to develop a vaccine against Ebola and the Middle East Respiratory Syndrome (MERS).
How their vaccine works: Sputnik V is a two-part adenovirus-based vector vaccine. It uses a vector (an engineered virus that lacks the gene to reproduce) to send a message in cells to produce spike proteins.
“The gene from adenovirus, which causes the infection, is removed while a gene with the code of a protein from another virus spike is inserted,” the institute explained.
“This inserted element is safe for the body but still helps the immune system to react and produce antibodies, which protect us from the infection,” it added.
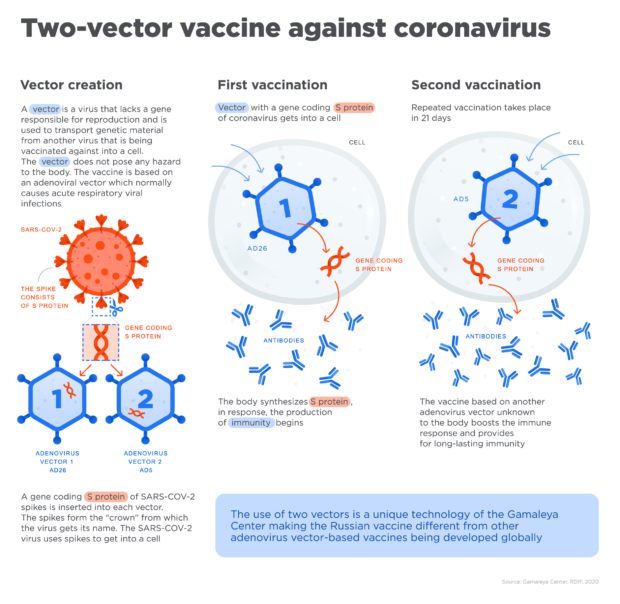
Photo courtesy of Gamaleya Center RDF
Sputnik V is stored in a regular refrigerator temperature (in dry form).
Status development: Gamaleya on November 24 announced that it has finished the second interim analysis of clinical trial data for Sputnik V.
READ: Russia vaccine ‘passes early trial test’
“The Sputnik V vaccine’s efficacy is confirmed at 91.4% based on data analysis of the final control point of clinical trials. The Sputnik V vaccine efficacy against severe cases of coronavirus is 100%,” Gamaleya reported.
In October, the Russian Direct Investment Fund (RDIF) submitted applications to the World Health Organization for an Emergency Use Listing and prequalification of Sputnik V.
According to a report on Monday, Russia is almost done completing its clinical trials for its COVID-19 vaccine for domestic animals and mink.
Issues and concerns: Some scientists have recently expressed concerns towards Sputnik V due to a “lack of safety data.” However, Russia denounced the criticisms.
Roll-out: Galvez recently said that the Russian vaccine may possibly be available in the Philippines as early as the first quarter of 2021.
COVAX Facility
COVAX Facility, compared to the first four companies on the list, offers a lower price for COVID-19 vaccines. Data showed that it will only cost around P854 for two doses per person. It can vaccinate at least 96,604,215 Filipinos, based on the information of Senator Angara.
About COVAX Facility: COVAX Facility, also known as the COVID-19 Vaccines Global Acces Facility, is a global mechanism designed to guarantee rapid, fair, and equitable access to COVID-19 vaccines worldwide.
It is co-led by Gavi, an international organization created to improve access to new and underused vaccines, along with the Coalition for Epidemic Preparedness Innovations and the World Health Organization.
The Philippines joined COVAX Facility in July.
How does COVAX Facility work? “The COVAX Facility will make investments across a broad portfolio of promising vaccine candidates (including those being supported by CEPI) to make sure at-risk investment in manufacturing happens now,” Gavi explained.
Since it follows a pooling process, COVAX can ensure rapid access to vaccines.
Status about COVID-19 vaccine: According to the Philippines’ vaccine czar Galvez, about 80 percent of the vaccines in the global market were already taken by rich countries. The COVAX Facility, on the other hand, was able to acquire 2 percent.
This means that the country is “fighting” to secure the other 18 percent of the vaccine supply.
AstraZeneca
AstraZeneca’s vaccine is worth only P610 for two doses per person. This appeared to be ideal for the country’s budget. Given its price and the P82.5 billion allocation of the government for vaccine procurement next year, it can inoculate 135,245,901 Filipinos, Angara’s graph indicated.
About AstraZeneca: AstraZeneca is a British-Swedish multinational pharmaceutical and biopharmaceutical company that focuses on the development and commercialization of prescription medicine.
Their medicines are targeted for the treatment of cardiovascular, metabolic, respiratory, inflammation, autoimmune, oncology, infection, and neuroscience diseases.
AstraZeneca collaborated with the University of Oxford to create a COVID-19 vaccine.
How their vaccine works: Like Russia’s Sputnik V, AstraZeneca’s vaccines use a genetically altered virus called adenovirus. This virus, which is not harmful to recipients, will carry spike protein (like the “crowns” in coronavirus). Injecting this, in theory, will help the immune system to recognize and attack coronavirus.
AstraZeneca’s vaccine requires a regular fridge temperature for storage.
Status development: AstraZeneca and the University of Oxford announced on December 8 that it finished the Phase 3 interim analysis for the vaccine.
The researchers noted that the efficacy rate for two doses – with the first dose at half strength – reached 90 percent. While a combination of two full-strength doses only resulted in 62 percent efficacy.
READ: AstraZeneca says COVID-19 ‘vaccine for the world’ can be 90% effective
Issues and concerns: Scientists questioned the vaccine efficacy rate after the reported error in dosage. In a report published by the New York Times on November 25, experts inquired how the combination of half dosage for the first shot and full dosage for the second shot resulted in a 90 percent efficacy rate.
Roll-out: According to the Department of Health (DOH), the government is negotiating with the firm, after AstraZeneca pulled out its application to conduct clinical trials in the country noting it already has enough data for the vaccine tests.
READ: Getting AstraZeneca vaccine still ‘doable’ even with company pullout from trial
“We are already having negotiations with AstraZeneca. So their next step would be the application for an emergency use authorization here in the country if ever and also with the other countries. So ito pong FDA (Food and Drug Administration) process na natin ang kanilang pag-a-applyan (This is the FDA process they have to undergo),” Health Undersecretary Maria Rosario Vergeire said on Monday.
Novavax
Among the seven vaccines in the data presented by Angara, Novovax was the most affordable. Two doses of Novavax vaccine costs only P366 per person, thus, some 225,409,836 Filipinos can receive and benefit from it amid the P82.5 billion budget.
About Novavax: Novavax, a late-stage US biotechnology company, focuses on the discovery, development, and commercialization of vaccines to prevent serious infectious diseases.
This Maryland company is smaller compared to other vaccine manufacturers. They received a $1.6 billion grant from the US government’s Operation Warp Speed to produce 100 million doses by 2021.
How their vaccines work: According to an article published in the Science Magazine, a peer-reviewed academic journal of the American Association for the Advancement of Science, Novavax first inserts baculovirus into moth cells.
The baculovirus, a pathogen that attacks insects, will make moth cells produce a protein called spike – which is present in coronavirus.
The spikes are then harvested by scientists and mixed with a “synthetic soap like particle” where the spikes can latch on. A compound called saponin, also derived from plants and trees, is also added to boost the immune response.
The NVX‑CoV2373 vaccine aims to produce anti spike antibodies that can block SARS-CoV-2 infection.
Status development: As of November 30, Novavax said in a statement that it has completed the enrollment of its 15,000-patient UK Phase 3 trial. The company added that has fully enrolled the Phase 2b trial in South Africa.
“Novavax expects its pivotal Phase 3 clinical trial in the United States and Mexico to begin in the coming weeks. More than 100 trial sites have been selected with some alternate sites in place, should they be needed,” the company added.
Roll-out: Since the vaccine is still in its early trial stages, there is still no expected release date.
However, the Indian pharmaceutical company Serum Institute of India (SII) previously committed to making the Novavax vaccine available in the country once it is developed.
KGA
For more news about the novel coronavirus click here.
What you need to know about Coronavirus.
For more information on COVID-19, call the DOH Hotline: (02) 86517800 local 1149/1150.
The Inquirer Foundation supports our healthcare frontliners and is still accepting cash donations to be deposited at Banco de Oro (BDO) current account #007960018860 or donate through PayMaya using this link.