Moscow starts mass COVID-19 vaccination with its Sputnik V shot
MOSCOW—Moscow began distributing the Sputnik V COVID-19 shot via 70 clinics on Saturday, marking Russia’s first mass vaccination against the disease, the city’s coronavirus task force said.
The task force said the Russian-made vaccine would first be made available to doctors and other medical workers, teachers and social workers because they ran the highest risk of exposure to the disease.
“You are working at an educational institution and have top-priority for the COVID-19 vaccine, free of charge,” read a phone text message received by one Muscovite, an elementary school teacher, early on Saturday and seen by Reuters.
Moscow, the epicenter of Russia’s coronavirus outbreak, registered 7,993 new cases overnight, up from 6,868 a day before and well above the daily tallies of around 700 seen in early September.
“Over the first five hours, 5,000 people signed up for the jab – teachers, doctors, social workers, those who are today risking their health and lives the most,” Mayor Sergei Sobyanin wrote on his personal website on Friday.
Article continues after this advertisement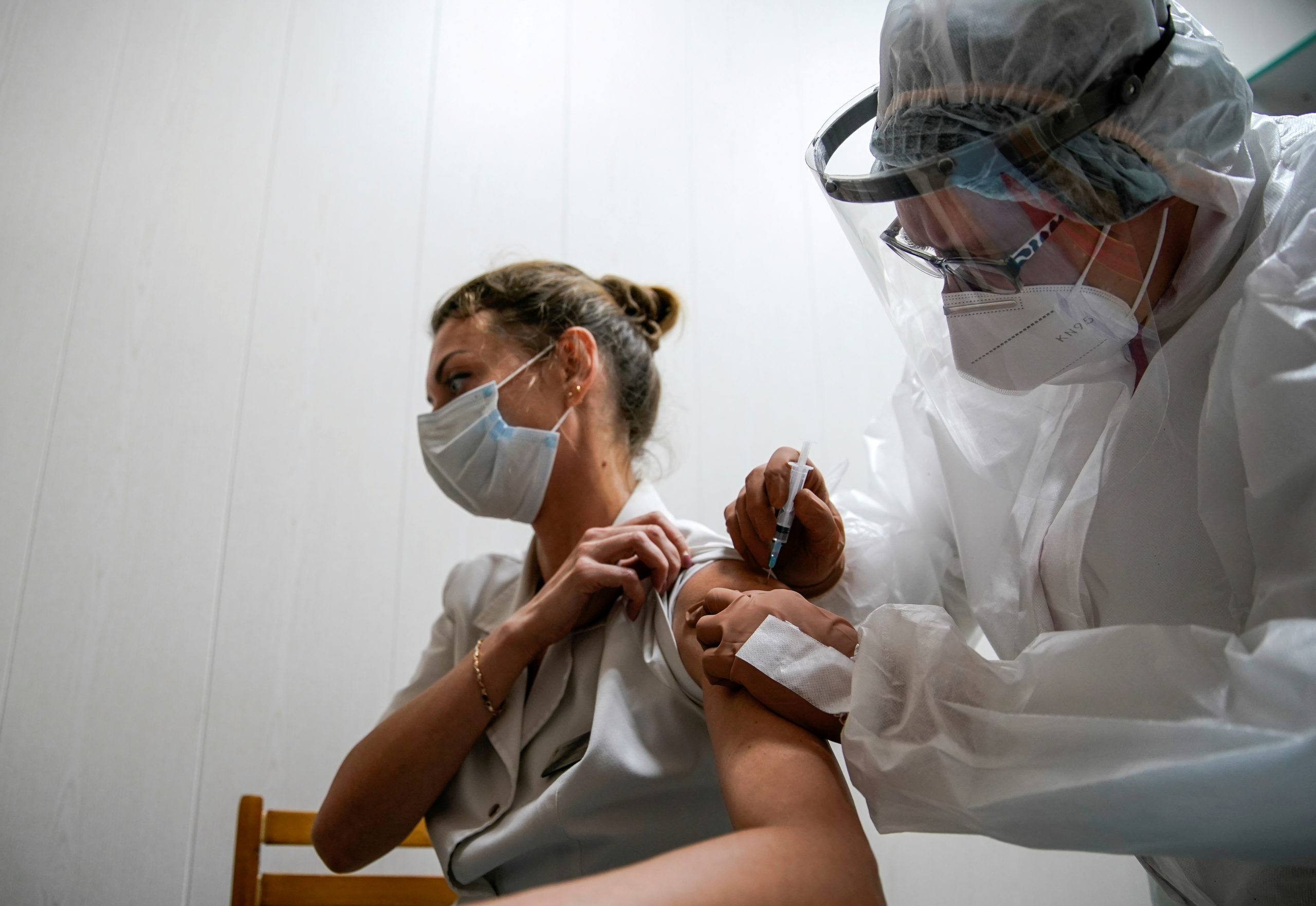
FILE PHOTO: A medic of the regional hospital receives Russia’s “Sputnik-V” vaccine shot against the coronavirus disease (COVID-19) in Tver, Russia October 12, 2020. REUTERS/Tatyana Makeyeva -/File Photo
The age for those receiving shots is capped at 60. People with certain underlying health conditions, pregnant women and those who have had a respiratory illness for the past two weeks are barred from vaccination.
Article continues after this advertisementRussia has developed two COVID-19 vaccines, Sputnik V which is backed by the Russian Direct Investment Fund and another developed by Siberia’s Vector Institute, with final trials for both yet to be completed.
Scientists have raised concerns about the speed at which Russia has worked, giving the regulatory go-ahead for its vaccines and launching mass vaccinations before full trials to test its safety and efficacy had been completed.
The Sputnik V vaccine is administered in two injections, with the second dose is expected to be given 21 days after the first.
Moscow closed down all public places including parks and cafes, with exception for delivery, in late March, with police patrolling the streets looking for whose violating the rules. Restrictions were eased from mid-June, however.
Russia as a whole reported 28,782 new infections on Saturday, its highest daily tally, pushing the national total to 2,431,731, the fourth-highest in the world.
In October, certain restrictions such as remote learning for some secondary school children and a 30% limit on the number of workers allowed in offices were introduced again.
For more news about the novel coronavirus click here.
What you need to know about Coronavirus.
For more information on COVID-19, call the DOH Hotline: (02) 86517800 local 1149/1150.
The Inquirer Foundation supports our healthcare frontliners and is still accepting cash donations to be deposited at Banco de Oro (BDO) current account #007960018860 or donate through PayMaya using this link.