2 makers of home test kits for COVID-19 have applied for special certification – FDA chief
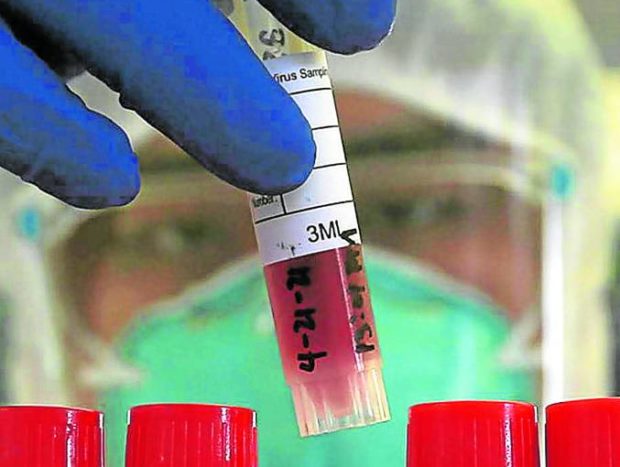
In this photo taken in April 2021, a health worker secures specimens collected at a COVID-19 testing site accredited by the Department of Health in Manila. So far only health care professionals are allowed to conduct testing for COVID-19. (File photo by RICHARD A. REYES / Philippine Daily Inquirer)
MANILA, Philippines — Two manufacturers of self-administered home test kits for COVID-19 have so far applied for special certification before the Food and Drug Administration (FDA), its acting director-general, Oscar Gutierrez, said in a taped briefing with President Rodrigo Duterte that started airing late Monday night.
The applications are currently being evaluated by the FDA and have been endorsed to the Research Institute for Tropical Medicine (RITM) for technical validation, Gutierrez said.
“Once the RITM finishes this, they can be given a special certification by the FDA,” he added.
Gutierrez reminded the public that the FDA had not yet approved self-administered COVID-19 test kits and all tests must be administered by health care professionals.
READ: DOH says no self-administered antigen test kit has been registered with FDA
Article continues after this advertisementSelf-administered test kits have different items and “special features” from test kits approved by the FDA — mostly that they do not have labels that they can be self-administered, he added.
Article continues after this advertisementThe FDA is so far urging manufacturers or product distributors or importers to apply to the FDA for a special certification so that these self-administered home test kits will be available in outlets.
READ: FDA accepts application for special certification of COVID-19 home test kits