Israel finds COVID-19 vaccine booster significantly lowers infection risk
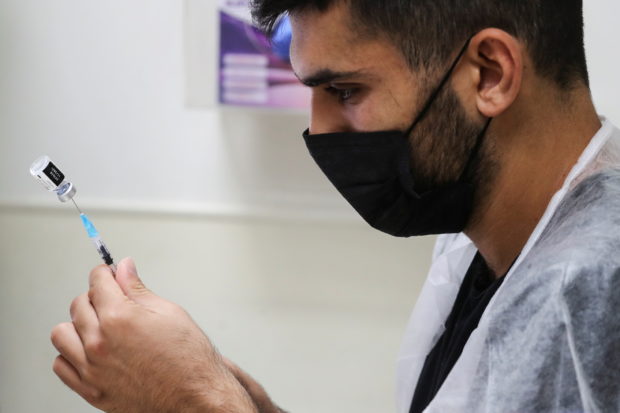
A medical worker prepares to administer a patient’s third dose of the coronavirus disease (COVID-19) vaccine as country launches booster shots for over 40-year-olds, in Jerusalem August 20, 2021. (REUTERS/Ammar Awad)
JERUSALEM – A third dose of Pfizer’s COVID-19 vaccine has significantly improved protection from infection and serious illness among people aged 60 and older in Israel compared with those who received two shots, findings published by the Health Ministry showed on Sunday.
The data were presented at a meeting of a ministry panel of vaccination experts on Thursday and uploaded to its website on Sunday, though the full details of the study were not released.
The findings were on par with separate statistics reported last week by Israel’s Maccabi healthcare provider, one of several organisations administering booster shots to try to curb the Delta coronavirus variant.
Breaking down statistics from Israel’s Gertner Institute and KI Institute, ministry officials said that among people aged 60 and over, the protection against infection provided from 10 days after a third dose was four times higher than after two doses.
A third jab for over 60-year-olds offered five to six times greater protection after 10 days with regard to serious illness and hospitalisation.
Article continues after this advertisementThat age group is particularly vulnerable to COVID-19 and in Israel was the first to be inoculated when the vaccine drive began in late December.
Article continues after this advertisementIn recent weeks, the health ministry has said immunity has diminished over time for seniors and younger people as well. Most vaccinated people who fell severely ill in Israel were over 60 and with underlying health conditions.
Israel started administering third jabs to over 60-year-olds on July 30. On Thursday it dropped the age of eligibility for a booster to 40, and included pregnant women, teachers and health care workers below that age. Third doses are given only to those who received their second shot at least five months ago.
The United States has announced plans to offer booster shots to all Americans, citing data showing diminishing protection. Canada, France and Germany have announced booster campaigns.
Fighting an outbreak of the Delta variant since June, Israel presently has one of the world’s highest infection rates per capita. Close to 1.5 million people out of the country’s 9.3 million population have taken a third jab.
gsg
For more news about the novel coronavirus click here.
What you need to know about Coronavirus.
For more information on COVID-19, call the DOH Hotline: (02) 86517800 local 1149/1150.
The Inquirer Foundation supports our healthcare frontliners and is still accepting cash donations to be deposited at Banco de Oro (BDO) current account #007960018860 or donate through PayMaya using this link.