COVID-19 vaccine may be ready by year-end—WHO
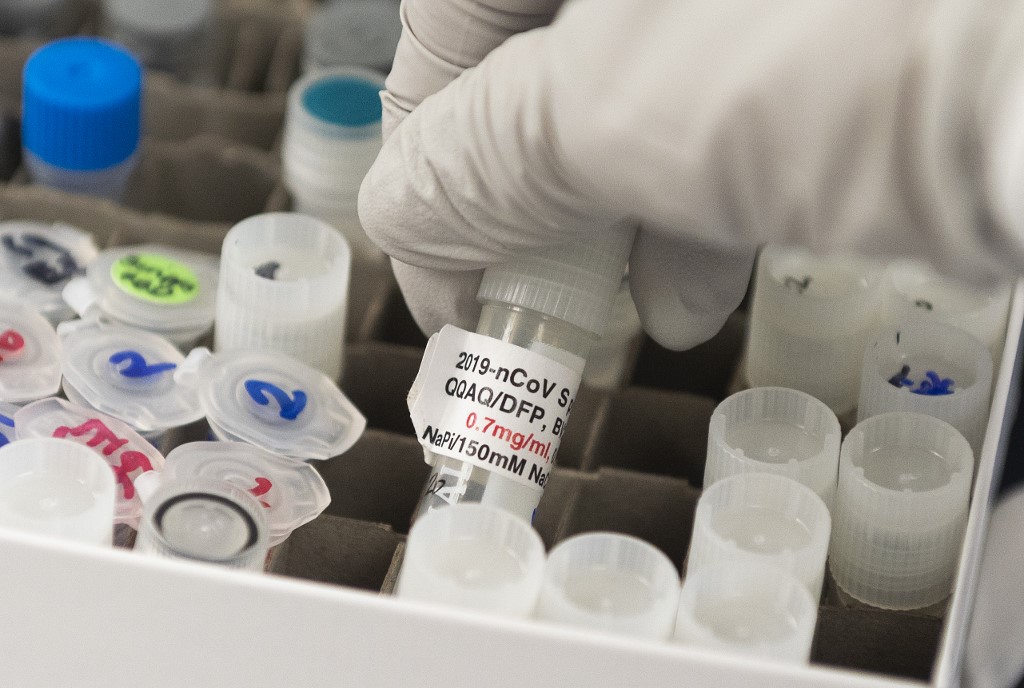
(FILES) In this file photo Dr. Nita Patel, Director of Antibody discovery and Vaccine development, lifts a vial with a potential coronavirus, COVID-19, vaccine at Novavax labs in Gaithersburg, Maryland on March 20, 2020, one of the labs developing a vaccine for the coronavirus, COVID-19. (Photo by ANDREW CABALLERO-REYNOLDS / AFP)
GENEVA— A vaccine against COVID-19 may be ready by year-end, the head of the World Health Organization (WHO) said on Tuesday, without elaborating.
WHO Director-General Tedros Adhanom Ghebreyesus, addressing the end of a two-day meeting of its Executive Board on the pandemic, said: “We will need vaccines and there is hope that by the end of this year we may have a vaccine. There is hope.”
Nine experimental vaccines are in the pipeline of the WHO-led COVAX global vaccine facility that aims to distribute 2 billion doses by the end of 2021.
For more news about the novel coronavirus click here.
What you need to know about Coronavirus.
For more information on COVID-19, call the DOH Hotline: (02) 86517800 local 1149/1150.
The Inquirer Foundation supports our healthcare frontliners and is still accepting cash donations to be deposited at Banco de Oro (BDO) current account #007960018860 or donate through PayMaya using this link.