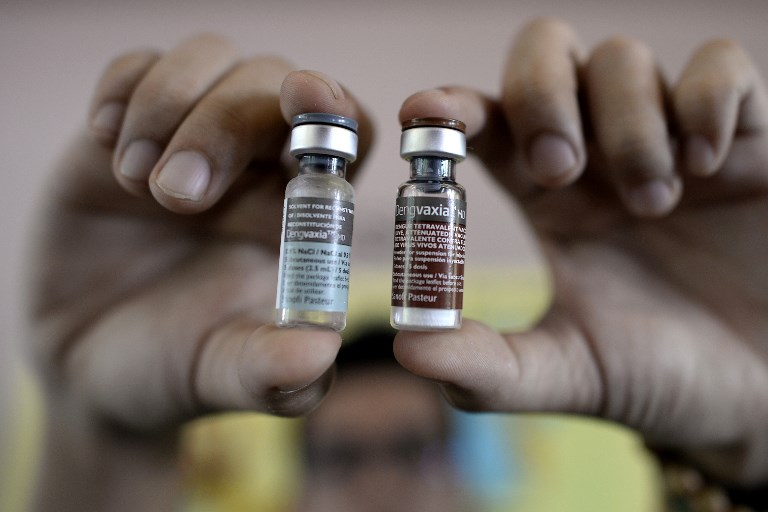
This photo, taken on April 4, 2016, shows a nurse showing vials of the dengue vaccine Dengvaxia, developed by French medical giant Sanofi Pasteur, during a vaccination program at an elementary school in Metro Manila. The Philippines said on Dec. 1, 2017 it had suspended use of the landmark vaccine for the potentially deadly dengue virus after its manufacturer warned it could worsen the disease in some cases. The Philippines has vaccinated more than 700,000 children with the drug since 2016 when it became the first country to start using it on a mass scale. Photo by NOEL CELIS / AFP)
The House public accountability committee chairman said it could reopen the hearing on the botched vaccination program if reports are true that the Dengvaxia vaccine has harmful effects on children.
Surigao del Sur Representative Johnny Pimentel, chairman of the committee on good government and public accountability, on Monday said that it can reopen the probe “if there is new information or there is a need.”
“The hearing about dengue vaccine has already been terminated but we can reopen it if there is new information or there is a need,” Pimentel said in a text message.
“We have to verify reports whether there were bad effects on the students, if it’s true then we will re-open the case,” he said.
Pimentel recalled that the previous House hearing on the anti-dengue vaccine, the French pharmaceutical firm Sanofi and the experts have certified that the vaccine was safe.
The Department of Health (DOH) said the vaccine was administered to than 700,000 children who had been vaccinated in Metro Manila, Calabarzon and Central Luzon.
Last Wednesday, Sanofi admitted that its Dengvaxia vaccine could cause more severe caseas of dengue for those who had not been previously infected.
The DOH has since suspended the vaccination program and ordered the close monitoring of everyone who had been vaccinated. /je