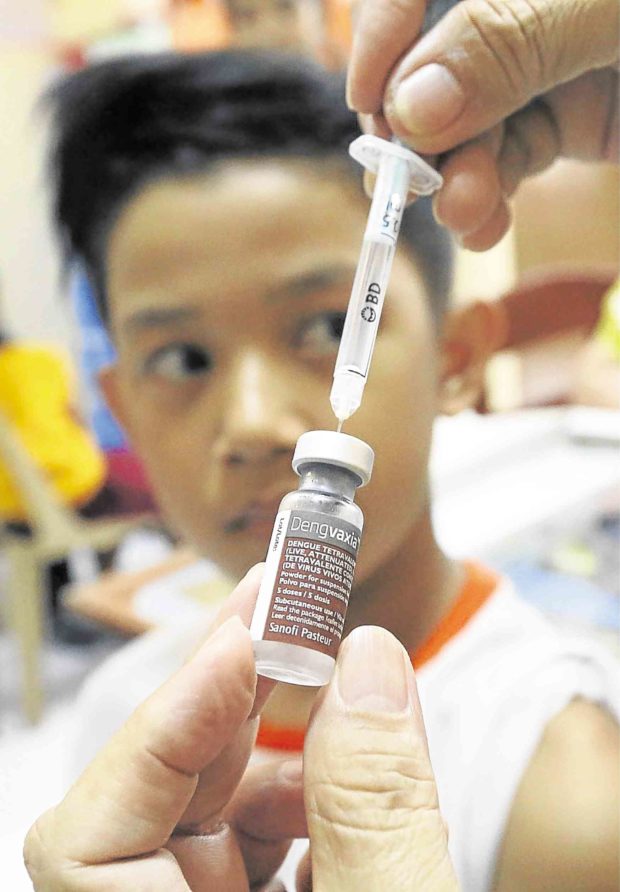
The P3.5-billion antidengue program placed high hopes on the Dengvaxia vaccine developed by French pharmaceutical company Sanofi. —MARIANNE BERMUDEZ
Vice President Leni Robredo on Sunday called on the government to look into the effects of a wrongly administered dengue vaccine on the health of hundreds of thousands of schoolchildren in the country and ensure that those who bungled the immunization program would be held liable.
Malacañang vowed to “leave no stone unturned” in the investigation of the fiasco that placed more than 733,000 schoolchildren at risk of severe dengue infection.
Justice Secretary Vitaliano Aguirre II said he would order the National Bureau of Investigation to look into the liability of government officials who had approved the purchase of Dengvaxia vaccine from the French pharmaceutical company Sanofi Pasteur.
The Department of Health (DOH) suspended the P3.5-billion immunization program on Friday, two days after Sanofi Pasteur announced that Dengvaxia could worsen the effects of dengue on people who had not been previously afflicted by the disease.
“This should be seriously studied and those who should be held accountable must be held accountable,” Robredo said on her weekly radio program.
What happens to the kids?
The “most important thing,” she said, was to “get the answers” to questions about the effects of Dengvaxia on the health of the schoolchildren who had received the vaccine.
“The parents of the children … they need to know what to do. If there really is a bad effect, what can be done to save their children?” Robredo said.
According to the DOH, more than 733,000 public school children aged 9 and above from Metro Manila, Central Luzon and Calabarzon (Cavite, Laguna, Batangas, Rizal, Quezon) have received at least one dose of the vaccine.
Presidential spokesperson Harry Roque said in a statement that those responsible for the use of Dengvaxia in the government’s antidengue drive would be held accountable.
“We will leave no stone unturned in making those responsible for this shameless public health scam, which puts hundreds of thousands of young lives at risk, accountable,” Roque said.
He called on the public to stay calm.
“We understand the concern of our people, especially the parents and the relatives of public elementary [school] children residing in [Central Luzon, Calabarzon and Metropolitan Manila], where the dengue vaccination initiative was launched by the previous administration,” he said.
“However, we call on all citizens not to spread information that may cause undue alarm,” he added.
DOH-DepEd monitoring
Roque noted that the DOH had not monitored any case of severe dengue infection among the children who received the vaccine last year.
He said the DOH was working with the Department of Education (DepEd) to monitor the health of the thousands of schoolchildren who had received Dengvaxia.
Aguirre said he had received a complaint from a parent whose son became ill after receiving the vaccine.
“One [parent] complained to me that his son, who was inoculated [with] that antidengue vaccine in April 2016, is now sick [with] baby TB (tuberculosis) and his immune system is now very weak,” Aguirre said in a text message to reporters.
He said he would order the NBI to investigate.
“Everybody who has some involvement will be included and appropriate charges will be filed against them if warranted,” he said.
WHO guidelines followed
In a text message to the Inquirer, former Health Secretary Janette Garin said the immunization program was implemented in line with World Health Organization (WHO) guidelines.
The dengue vaccination program was launched during Garin’s term.
“Implementation was based on recommendations from both local and global experts,” Garin said.
She said dengue, which was affecting 90 to 93 percent of the population in the recommended and targeted areas “was [dealt with] as our government’s obligation to respond.”
On Friday, Health Secretary Francisco Duque announced the suspension of the immunization program after Sanofi Pasteur said Dengvaxia was safe and effective in the long run only for people who had been infected before they were vaccinated.
“For those not previously infected by dengue virus, however, the analysis found that in the longer term, more cases of severe disease could occur following vaccination upon a subsequent dengue infection,” the pharmaceutical company said in a statement.
Duque, however, was quick to ease fears of parents who were worried about their children who had been vaccinated, particularly those who had no prior infection.
He assured them that the DOH was on top of the situation.
“The vaccine has a 30-month protection period against dengue, regardless [of whether] the child had prior infection or not,” he said.
Duque added that the DOH would monitor the health of all the recipients of the vaccine.
Review going on
He said a review of the program was going on, including consultation with experts and the WHO.
The WHO’s Strategic Advisory Group of Experts on Immunization will soon meet to discuss the latest development on the dengue vaccine, he said.
Concerns about the effects of Dengvaxia on people without prior infection were raised by resource persons in an inquiry called by the good government committee of the House of Representatives last year.
At a hearing on Nov. 29, 2016, Antonio Dans of the Philippine College of Physicians expressed worry that antibodies introduced by the vaccine might “enhance” the dengue virus if a person is bitten by the mosquito after immunization.
Dans told lawmakers that the immunization program should be suspended until the theory of antibody dependent enhancement was thoroughly studied.
But Sanofi Pasteur’s regional dengue expert, Anh Wartel, played down the possibility, saying “the concerns expressed in the background are based upon ‘what-if’ conjectures.”
She said the chance of the scenario unfolding would not be great because the high “seroprevalence”—the number of infected people in a given population—was rather high in the regions where the vaccine was administered.
Undue haste
Besides the vaccine’s effects, the House inquiry also tackled the alleged undue haste in the DOH’s procurement of Dengvaxia shortly after the May elections in 2016.
The Food and Drug Administration approved the certificate of product registration on Dec. 22, 2015; a month later, on Jan. 21, 2016, the Philippine Children’s Medical Center made a purchase request.
The Formulary Executive Council issued a certification for Dengvaxia on Feb. 3, 2016. The certification is a requirement for medical items not included in the Philippine National Drug Formulary.
The immunization program started on April 4, 2016, two months before the Aquino administration ended.