Pharma, drug store ordered to stop dengue immunization services
The Food and Drug Administration (FDA) has ordered a pharmaceutical company and a drug store chain to stop promoting and offering immunization services without authorization, as such activities could pose “a potential health hazard to the consuming public.”
The FDA asked Sanofi Pasteur Inc. and Watson’s Personal Care Stores to explain why administrative and criminal cases should not be filed against them for conducting immunization activities using Sanofi’s dengue vaccine, Dengvaxia.
In a statement, FDA Director General Nela Charade Puno said the agency had not issued any authorization to allow immunization services to be conducted by Sanofi, Watson’s or any drug store.
“There is no assurance on the safety and quality of the service rendered in these drugstores and thus [it] poses a potential health hazard to the consuming public,” Puno said.
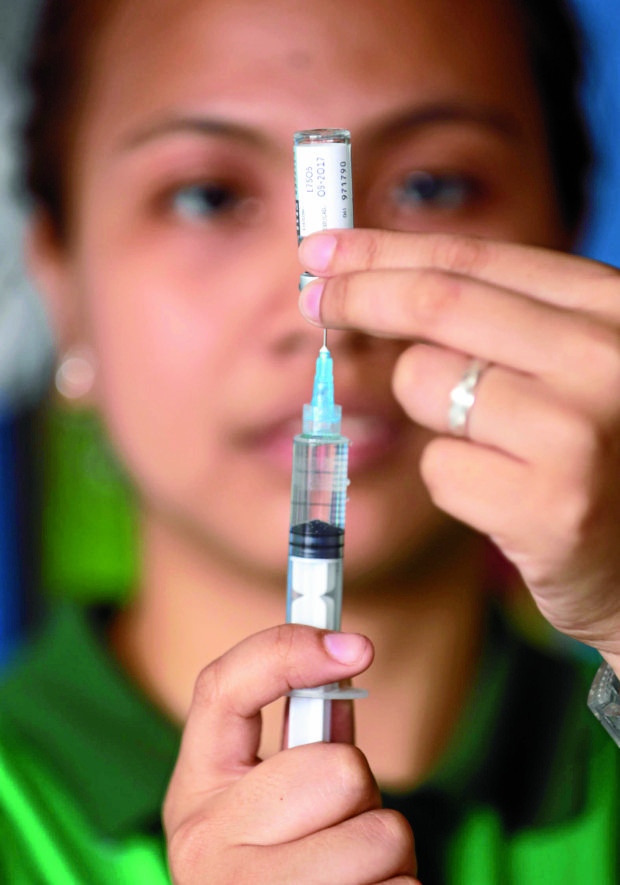
The FDA’s cease and desist order was issued following Watson’s announcement that it would conduct immunization activities at the drug store using the vaccine which cost P4,000 a dose. Three doses have to be administered in a year.
Unauthorized ads
In April, the FDA’s Center for Drug Regulation and Research monitored Watson’s unauthorized advertisement and promotion of the vaccine, and noted that the drug store chain had misrepresented Dengvaxia as available over the counter. The vaccine is classified as a prescription drug approved only for those 9 to 45 years old.
The FDA said it would similarly ask Sanofi to explain why it violated the law for the second time. In December last year, the agency issued a cease and desist order and filed a case against the pharmaceutical firm for the unauthorized promotion of Dengvaxia.
Puno warned that if the FDA finds Sanofi’s explanation unsatisfactory, or if the firm continued to commit violations, the agency would suspend or cancel the vaccine’s certificate of product registration.
“Laws are mandatory and they are meant to be followed,” the FDA chief said.
“Compliance with the law is not optional, especially when the safety and health of the public are involved,” she added. “Sanofi, Watson’s and other manufacturers and drug stores are hereby warned. Nobody is exempt [from the law].”
The FDA chief also advised the public against availing themselves of unauthorized immunization services, and warned drug stores against conducting such activities until the appropriate regulation and authorization had been issued.
Strictly pursued
“Regulation action and sanctions shall be strictly pursued in case of noncompliance,” Puno said.
For a drug store to be able to undertake additional activities such as vaccination services, it must apply for a variation of its license to operate from the FDA and comply with its requirements, among them patient counseling, having a qualified person to administer the vaccine, dedicated space and sanitation.
The FDA and the Department of Health train and authorize FDA-licensed community pharmacists to administer adult vaccines and other immunization products.
The agency also urged the public to report unauthorized immunization activities in drug stores via an e-mail to report@FDA.gov.ph, or at the FDA’s online reporting facility at www.fda.gov.ph.