Party balloons and science
Decorative balloons are used at birthdays and other celebrations. Coming in different colors, sizes and shapes, they are made either from natural latex from rubber trees or synthetic latex.
What does a balloon have in common with a lunch box, a basketball and DNA (deoxyribonucleic acid, the hereditary material in humans and almost all other organisms)? They are all polymers.
Polymers consist of many molecules bound together to form long chains.
How things made of polymers look, feel and act depend on how their atoms and molecules are connected. Some are sticky and gooey, some rubbery like a ball, and some are hard and solid.
Polymers are both man-made and naturally occurring. They can return to their initial state after being stretched.
This characteristic is called elasticity.
Whenever we inflate and deflate balloons, we see elasticity at work. The polymer can stretch its chains of molecules then return them to their original compact state.
Did you know?
The world’s first novelty and printed balloon was made by Neil Tillotson, a chemical engineer. Frustrated that he could not create inner tubes from liquid latex, he came up with another idea that turned out to be an amusing novelty.
He shaped a piece of cardboard into a cat’s head complete with ears and dipped it in latex. He then sprinkled the dried latex with talc to keep the rubber from sticking. After rolling the latex off the cardboard, he put it to his lips and blew some air into the hole at the bottom. He kept blowing until the latex was round and taut. And that was how balloons came to be.
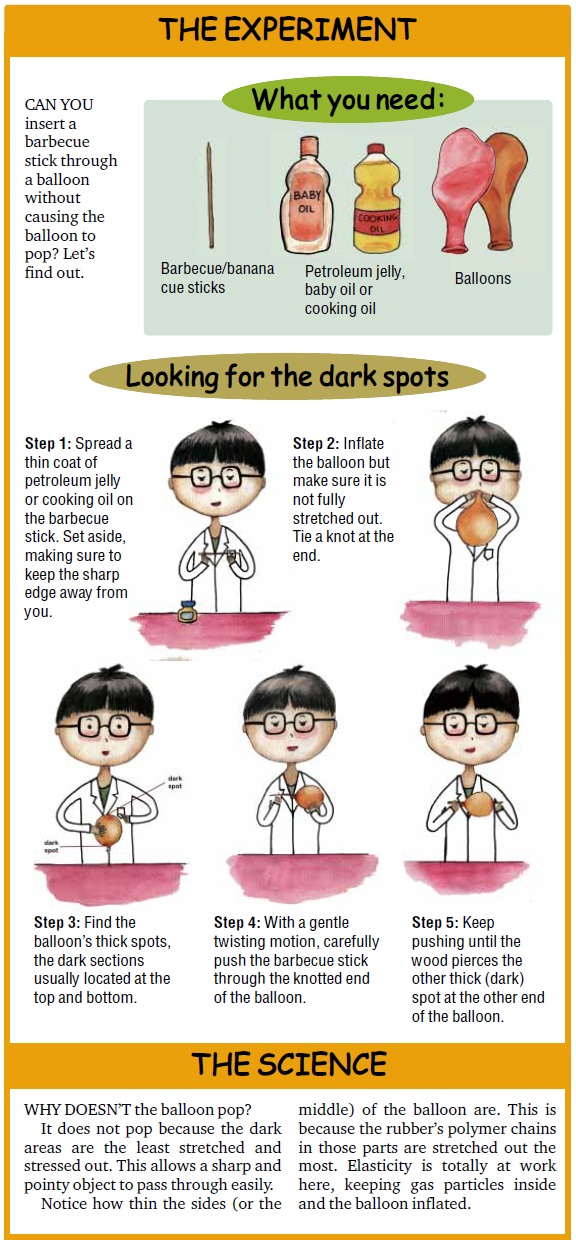
The Experiment
Can you insert a barbecue stick through a balloon without causing the balloon to pop? Let’s find out.
What you need:
Balloons
Barbecue/banana cue sticks
Petroleum jelly, baby oil or cooking oil
Looking for the dark spots
Step 1: Spread a thin coat of petroleum jelly or cooking oil on the barbecue stick. Set aside, making sure to keep the sharp edge away from you.
Step 2: Inflate the balloon but make sure it is not fully stretched out. Tie a knot at the end.
Step 3: Find the balloon’s thick spots, the dark sections usually located at the top and bottom.
Step 4: With a gentle twisting motion, carefully push the barbecue stick through the knotted end of the balloon.
Step 5: Keep pushing until the wood pierces the other thick (dark) spot at the other end of the balloon.
The Science
Why doesn’t the balloon pop?
It does not pop because the dark areas are the least stretched and stressed out. This allows a sharp and pointy object to pass through easily.
Notice how thin the sides (or the middle) of the balloon are. This is because the rubber’s polymer chains in those parts are stretched out the most. Elasticity is totally at work here, keeping gas particles inside and the balloon inflated.
Takeaway learning
Polymers play an essential role in everyday life. They are used to manufacture food containers, phone casings, bottles, clothes, computers, medical equipment and many things used every day.
You can maximize the use of polymers by recycling food containers and bottles. They may be used as plant containers, for instance, or turned into decorative items. When they have to be disposed of, they should be put in the nonbiodegradable bin.
About the series
The joy of discovery. The fun of learning.
These are the experiences that Bayer wants to pass on to young people in keeping with its mission, “Bayer: Science for a Better Life,” and its enthusiasm for research.
By putting together simple, educational and fun experiments, elementary school students are encouraged to learn science by doing science.
The Bayer Smiling Kiddie Einsteins series offers teachers, parents and students hands-on and inquiry-based experiences that involve observing, experimenting, hypothesizing, analyzing and testing.
Through this series of experiments related to health, agriculture-nutrition and hi-tech materials, Bayer and Inquirer in Education aim to deepen the interest of elementary pupils in science.
The materials needed for these experiments are safe and can easily be accessed from your homes.
Using the newspaper
Look in the Sports section. Polymers are used in sports because they are materials that can bend and twist, stick to each other and get tangled up, making things stronger and easier to grip.
Make a list of the sports that you can read about in today’s issue. Then make a list of the equipment that are needed by each sport.
Make two other lists. One list should be of all the team sports that you can find in this section. The other list should be of the individual sports that are in today’s news.