MANILA, Philippines—The Food and Drug Administration (FDA) warned the public Saturday not to use an unregistered eye drop being sold in the market that claims to be good for a host of eye ailments but has not passed muster.
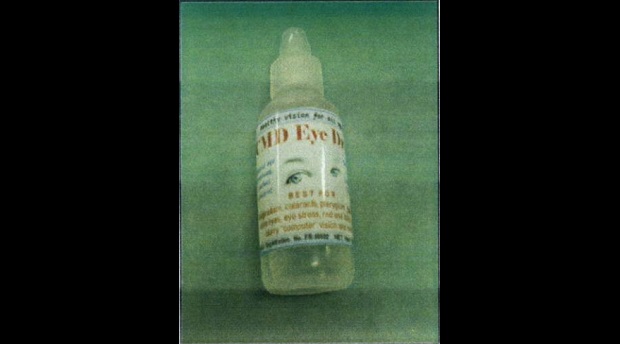
In an advisory, the FDA said CMD Eye Drop bears the label “BFAD-registered” but is actually unregistered with the agency. The initials stand for Bureau of Food and Drugs, the FDA’s old name
The FDA stressed that the use of the unregistered eye drops may result in inflammation, irritation, infection of the eyes or loss of vision.
Labeled as “best for astigmatism, cataracts, pterygium, glaucoma, sore eyes, eye stress, red and itchy eyes, blurry ‘computer’ vision and more,” CMD Eye Drop is reportedly being sold in Metro Manila at P120 per 15 ml bottle and in refill bottles in the provinces.
“The FDA warns the public that the use of unregistered eye drops presents safety risks and adverse health consequences. Since eye drops are to be applied directly to the eye area, the preparation of these eye solutions requires special consideration and should only be dispensed in special containers,” the advisory said.
The FDA ordered its inspectors to seize unregistered CMD Eye Drops from all outlets and establishments where they are sold.
The health agency also advised consumers to use only FDA-registered health products and to look for the FDA registration number or DR number when drug products are involved.
RELATED STORIES
Abbott recalls stinking antiplatelet drugs
FDA warns online sellers of banned slimming pills