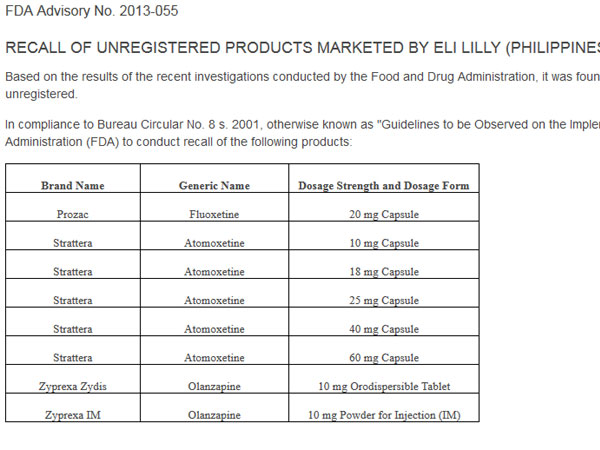
MANILA, Philippines—The Food and Drug Administration (FDA) has ordered the recall of some drugs used to treat mental and behavioral disorders after these were found to be unregistered.
In an advisory posted on its website, the FDA ordered the recall of Prozac (Fluoxetine), an antidepressant drug, and Strattera (Atomoxetine), which is used to treat Attention Deficit Hyperactivity Disorder (ADHD).
No valid certificates
The FDA also ordered the recall of Zyprexa Zydis (Olanzapine) orodispersible tablets and Zyprexa IM (Olanzapine) powder for injection (IM), which are used to treat symptoms of psychotic conditions such as schizophrenia and bipolar disorder.
The FDA said the products were being marketed by Eli Lilly (Philippines) Inc. “without valid certificates of product registration (CPR), hence they are unregistered.”
The FDA warned physicians against prescribing the drugs. It also advised the public to refrain from buying and using the products.
“All FDA inspectors are hereby directed to ensure that these products will not be found in the warehouses of Eli Lilly distributors and in retail drugstores after Eli Lilly has concluded the recall process,” the FDA said in its advisory.—Tina G. Santos