(Editor’s note: We are posting this news item again in connection with a new post on this fake and unauthorized product. This serves as a warning to the public)
MANILA, Philippines–A social media post has been making rounds claiming the Department of Health has officially announced the release of a “revolutionary” diabetes support product.
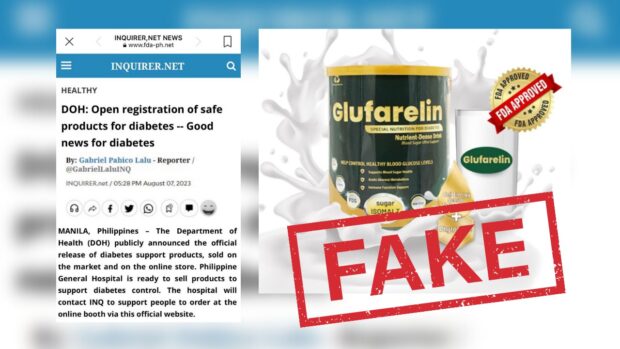
The post, titled “DOH: Open registration of safe products for diabetes–Good news for diabetes,” falsely claims to be posted on INQUIRER.net, even imitating the news website’s logo and signature color.
These claims are not only misleading but could also pose a threat to public health.
What does the post claim?
The post promotes “Glufarelin Gold” as a groundbreaking therapeutic product capable of normalizing glucose levels. By allegedly stabilizing blood sugar swiftly, the post suggests that patients can comfortably manage diabetes over extended periods.
“This new therapeutic product has the potential to return glucose levels to normal. Stabilizing blood sugar quickly along with knowing how to control diabetes, patients can completely live peacefully with it for a long time,” it said.
It even goes as far as asserting that the product is available for sale at the Philippine General Hospital.
FDA’s standpoint
Contrary to the claims made in the post, the Food and Drug Administration (FDA) has explicitly warned the public against purchasing or using “Glufarelin Gold Special Nutrition for Diabetes 400g.”
It said that “Glufarelin Gold” has not complied with the required registration process with the agency and lacks the essential Certificate of Product Registration, crucial for verifying the product’s quality and safety. It did not undergo safety and efficacy tests.
The FDA cautions the public “against the purchase and use of the unregistered drug product with false therapeutic claims,” adding that the consumption of such a product “might expose consumers to potential health risks or injuries.”
Legal implications
Under Republic Act No. 9711, or the “Food and Drug Administration Act of 2009,” it’s illegal to manufacture, import, export, sell, offer for sale, distribute, transfer, promote, advertise, or sponsor health products that lack FDA’s proper authorization.
Establishments or entities distributing “Glufarelin Gold” without appropriate permissions are liable to face stringent regulatory actions and sanctions, according to the FDA.
The public is strongly advised to utilize the FDA Verification Portal at verification.fda.gov.ph to ascertain the registration status of any health product before purchase.