MANILA, Philippines—The US Food and Drug Administration (FDA) has approved the use of a vaccine against the respiratory syncytial virus (RSV)—a respiratory disease that sends over 177,000 elderly people in the US to hospitals each year, and is also common among Filipino children.
Multinational pharmaceutical and biotechnology company GSK’s Arexvy vaccine on May 03 was announced to be the first RSV vaccine approved for use in the US. The FDA approved the use of Arexvy for the prevention of lower respiratory tract disease caused by RSV in individuals 60 years of age and older.
“Older adults, in particular those with underlying health conditions, such as heart or lung disease or weakened immune systems, are at high risk for severe disease caused by RSV,” said FDA’s Center for Biologics Evaluation and Research Director Dr. Peter Marks.
“Today’s approval of the first RSV vaccine is an important public health achievement to prevent a disease which can be life-threatening and reflects the FDA’s continued commitment to facilitating the development of safe and effective vaccines for use in the United States,” Marks said.
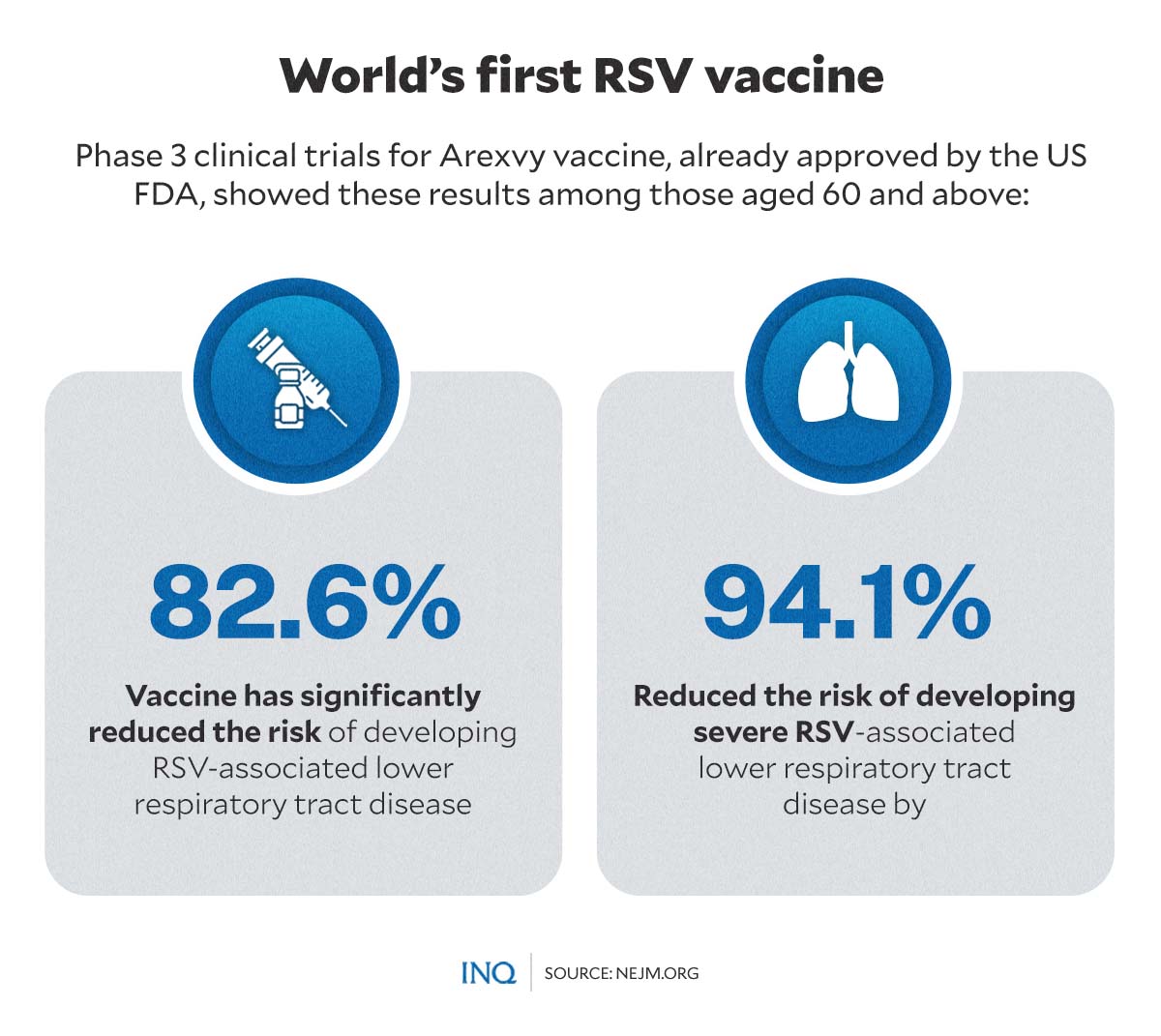
The FDA approved the vaccine following an analysis of data from the phase 3 clinical trials for Arexvy, which was submitted by GSK.
Data showed that Arexvy vaccine has significantly reduced the risk of developing RSV-associated lower respiratory tract disease by 82.6 percent and reduced the risk of developing severe RSV-associated lower respiratory tract disease by 94.1 percent among people aged 60 and older.
The results of the clinical trial were published in The New England Journal of Medicine.
RSV: A common and deadly disease
According to the US Centers for Disease Control and Prevention (CDC), RSV is a common respiratory virus, which usually causes mild cold-like symptoms—and can be a serious disease, especially among infants and the elderly.
It is estimated that between 60,000-160,000 older adults in the United States are hospitalized and 6,000-10,000 of them die each year due to RSV infection. Among the adults who are at highest risk for severe RSV infection include:
- older adults who are at least 65 years and older
- adults with chronic heart or lung disease
- adults with weakened immune system
The US CDC also stressed that from 58,000-80,000 children younger than 5 years old are hospitalized in the US due to RSV infection. Premature infants, infants aged 6 months and younger, and children younger than 2 years old with chronic lung disease or congenital disease are among those at greatest risk for severe illness from RSV.
“Virtually all children get an RSV infection by the time they are 2 years old. Most of the time RSV will cause a mild, cold-like illness, but it can also cause severe illness such as bronchiolitis (inflammation of the small airways in the lung) and pneumonia (infection of the lungs),” said US CDC.
In the Philippines, the Department of Health (DOH) said there were 221 cases of RSV among Filipino children from January 1 to August 31 last year. No deaths have been reported due to RSV, the DOH said.
Common symptoms
The health department explained that although the disease affects mostly children below 5 years of age, it can be transmitted through respiratory droplets and can infect adults and seniors.
“This is contagious even to seniors … because this [illness] is airborne. We need to make our kids healthy and vaccinated to stay protected and keep the vulnerable population safe,” said Health Undersecretary Maria Rosario Vergeire, DOH officer in charge.
Symptoms of RSV usually show within 4 to 6 days after infection. Common symptoms of RSV infection include:
- runny nose
- decrease in appetite
- coughing
- sneezing
- fever
- wheezing
“These symptoms usually appear in stages and not all at once. In very young infants with RSV, the only symptoms may be irritability, decreased activity, and breathing difficulties,” the US CDC said.
“Most RSV infections go away on their own in a week or two,” it added.