DOH eyes price cap for COVID self-test kits
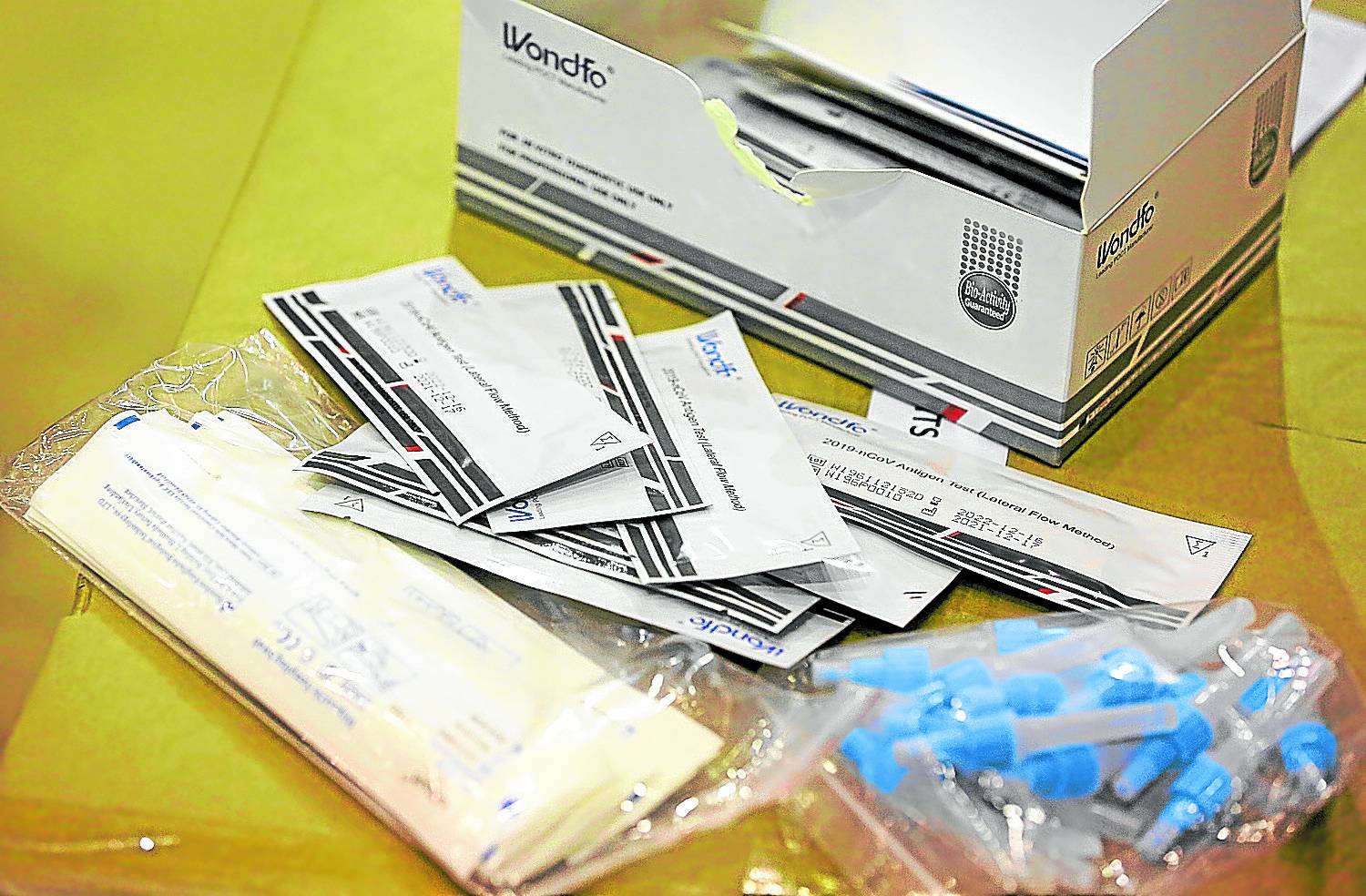
SELF-TEST Antigen test kits provide faster results than the RT-PCR tests, considered the gold-standard in COVID-19 testing. —NIñO JESUS ORBETA
MANILA, Philippines — Following the Food and Drug Administration’s (FDA) approval of two self-administered COVID-19 antigen test kits, the Department of Health (DOH) said it was eyeing to release this week guidelines for the use of the kits but that it might still need a week or two before it could issue a price cap.
On Monday, the FDA approved two brands of self-administered antigen test kits: Panbio COVID-19 Antigen Self-test of Abbott Laboratories Philippines, an affiliate of the American multinational company of the same name; and the SARS-CoV-2 Antigen Rapid Test of Chinese company Labnovation Technologies Inc.
Health Undersecretary Maria Rosario Vergeire on Wednesday said the DOH will release a policy stating the guidelines for the use of the self-administered antigen test kits either on Thursday or Friday.
She said the guidelines would include instructions on reporting results to local governments, which would then report to the DOH.
‘Chat box’
According to Vergeire, the health agency is also exploring its current system where citizens use a “chat box” to report on the results of these self-test kits.
“We are trying to look at this system if it still has the capacity so that citizens using self-administered test kits will be able to chat in their use and we can be able to get the results and have a sense of how many are being used for these self-administered test kits at home,” she said.
Vergeire also said the DOH would discuss with the Department of Trade and Industry the possibility of a price cap on such products, adding that they might need one to two more weeks to study the price range before issuing a price cap for home antigen test kits.
With the approval of self-administered test kits, acting presidential spokesperson and Cabinet Secretary Karlo Nograles is optimistic that the cost of reverse transcription-polymerase chain reaction (RT-PCR) testing will go down.
In December, the DOH set a price cap of P2,800 for plate-based RT-PCR tests and P2,450 for GeneXpert tests in public laboratories. The price cap for private laboratories was set at P3,360 for plate-based and P2,940 for GeneXpert tests.
“Right now, we have self-administered testing kits and, with improved technologies, perhaps we might hopefully see a decrease in the cost of testing kits. If people have more options, the prices of commodities will adjust,” he added.
Nograles said the Inter-Agency Task Force for the Management of Emerging Infectious Diseases already had two meetings on the rate of RT-PCR testing, but was amenable to have another round of talks to further lower the price cap for COVID-19 testing in the country.
For more news about the novel coronavirus click here.
What you need to know about Coronavirus.
For more information on COVID-19, call the DOH Hotline: (02) 86517800 local 1149/1150.
The Inquirer Foundation supports our healthcare frontliners and is still accepting cash donations to be deposited at Banco de Oro (BDO) current account #007960018860 or donate through PayMaya using this link.














