DOH warns vs improper use of antigen test kits
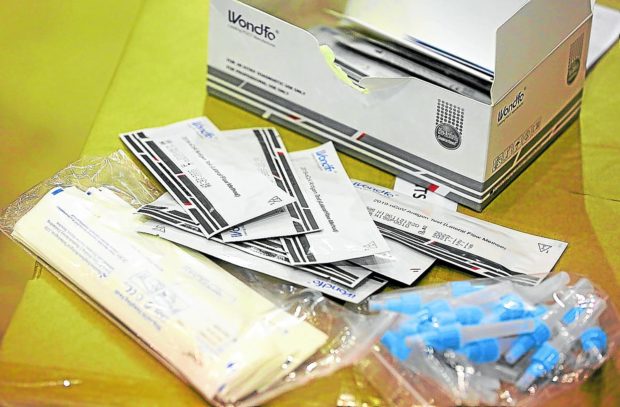
SELF-TEST Antigen test kits provide faster results than the RT-PCR tests, considered the gold-standard in COVID-19 testing. —NIñO JESUS ORBETA
MANILA, Philippines — The Department of Health (DOH) on Saturday warned the public on the purchase and use of self-administered COVID-19 test kits, which are now in the final stages of evaluation by the Food and Drug Administration (FDA).Health Undersecretary Maria Rosario Vergeire said in a radio interview that the DOH will soon release guidelines on how the self-administered antigen test kit products should be used.
“We are drafting the guidelines on how these self-administered test kits will be used,” she said. The antigen test kits currently available in the market should be used with the guidance of health-care professionals to avoid false results.
“The reliability of antigen test kits will depend on its use. The most accurate result of an antigen test comes if you use it at the right time and appropriate use,” she said.
“The antigen tests can only be used if a person has symptoms, and this is within the first five days of illness. That’s when the results are most accurate,” Vergeire said.
Dubious kits
Vergeire also warned consumers against using dubious kits that make false claims, citing products that claim to be approved by the Research Institute for Tropical Medicine (RITM).
She said the RITM does not approve test kits and it was only the FDA that issues the approval for such products.
Vergeire explained that only the FDA receives applications, which will then undergo an initial evaluation before they are passed on to the RITM, which validates the efficacy of medical devices, like testing kits.
The RITM would then determine whether the test kits meet the required specificity of 97 percent and sensitivity of 80 percent in identifying only the virus causing COVID-19.
“This takes four to five days with RITM and then will be returned to the FDA. So, all in all, the process can take up to seven days,” she added.
No approved self-test kit
“What we need to look for in the labels should be the registration of FDA. This means that it has been certified by the FDA already. RITM is just part of the process of evaluation by the FDA. But it’s the FDA that issues the registration,” Vergeire explained.
As of now, she said the FDA had yet to approve any self-administered test kits in the country.
The health agency earlier said that at least 11 manufacturers of self-administered COVID-19 test kits had applied for FDA approval of their products.
For more news about the novel coronavirus click here.
What you need to know about Coronavirus.
For more information on COVID-19, call the DOH Hotline: (02) 86517800 local 1149/1150.
The Inquirer Foundation supports our healthcare frontliners and is still accepting cash donations to be deposited at Banco de Oro (BDO) current account #007960018860 or donate through PayMaya using this link.














