
Artwork by Daniella Marie Agacer
MANILA, Philippines—The last few days were reminiscent of 2020, when pharmacies had a shortage of medical masks as the Philippines confirmed its first COVID-19 case and people’s fear of contracting the disease was escalating.
This year, however, it’s not medical masks but paracetamol and analgesic brands that are commonly used to ease fever and mild to moderate pain.
Unilab Inc., a Filipino pharmaceutical firm, said on Jan. 4 that some of its brands were temporarily out of stock in select outlets, especially in Metro Manila, because of “extraordinary demand.” It said sorry for the shortfall.
Why the demand?
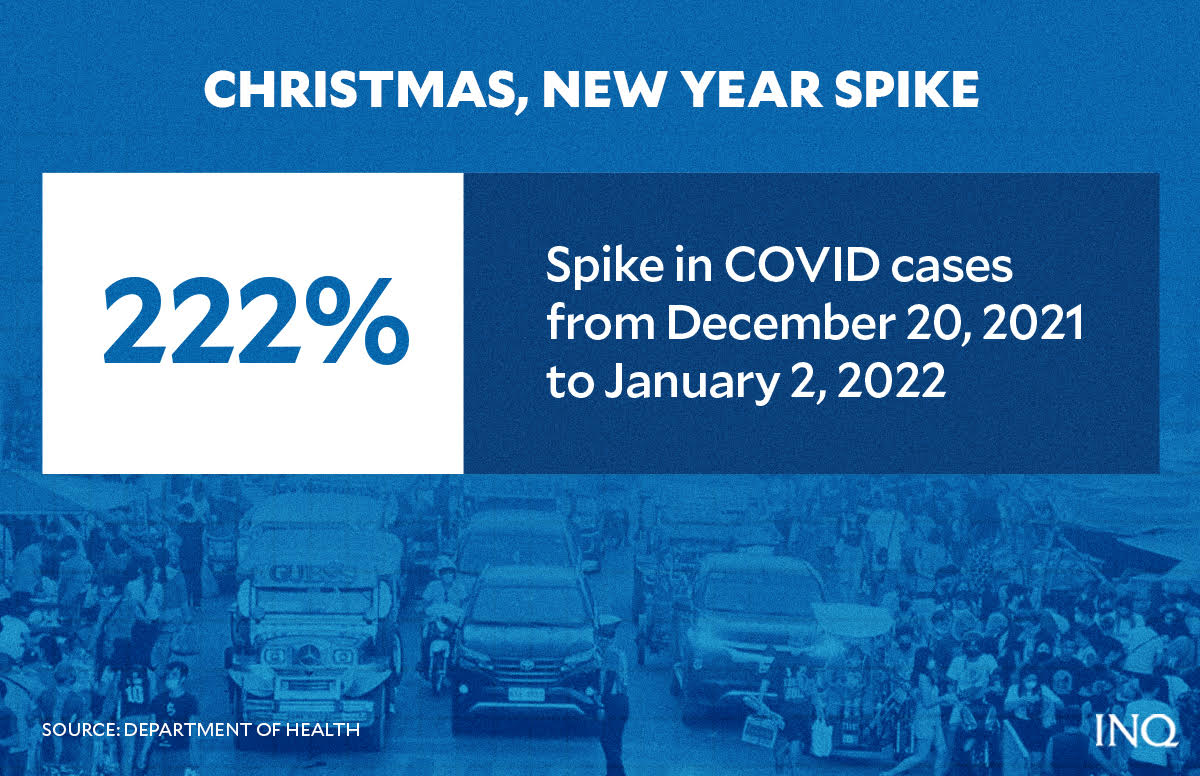
On Jan. 3, the Department of Health (DOH) again declared that the country was at high risk of COVID because of an increase in cases during Christmas and New Year celebrations when social distancing was thrown under the rug.
Graphic by Ed Lustan
Health Undersecretary Ma. Rosario Vergeire said there was a 222 percent increase in new cases when comparing those recorded from Dec. 6 to 9, 2021 and Dec. 20, 2021 to Jan. 2, 2022. Although she described it as moderate, the daily virus attack rate was 1.07 for every 100,000 individuals.
The Philippines was seeing a steady decline in COVID-19 cases in December, but the independent monitoring group Octa Research said cases could rise because of physical gatherings and threats of Omicron, a more contagious variant of SARS Cov2, the virus that causes COVID-19.
Last Dec. 20, there were only 263 new COVID-19 cases; 168 on Dec. 21; 261 on Dec. 22; 288 on Dec. 23; 310 on Dec. 24; 433 on Dec. 25; 433 on Dec. 26; 318 on Dec. 27; 421 on Dec. 28; 889 on Dec. 29; 1,623 on Dec. 30; 2,961 on Dec. 31; 3,617 on Jan. 1; and 4,600 on Jan. 2.
Graphic by Ed Lustan
The Pharmaceutical and Healthcare Association of the Philippines (PHAP) explained that the shortage in paracetamol brands, which it said was temporary, was driven by heightened vigilance against Omicron and the rise in the number of people getting sick for different reasons.
READ: Group: Some areas having ‘temporary shortage’ of paracetamol brands
Raul Ting, a doctor in Tuguegarao City, said because of the rise in COVID-19 cases, people, out of fear, will cling to anything that they believe will help them prevent or handle sickness.
He told INQUIRER.net that this kind of shortage was also seen in Tuguegarao City in August 2021, when medicines for colds went out of stock as the city saw a serious increase in infections.
Graphic by Ed Lustan
Kristine Doloroso, also a doctor, told INQUIRER.net that since Metro Manila reverted back to Alert Level 3 because of the rise in COVID-19 cases, “we are currently experiencing panic among our people.”
“The anxiety caused people to panic-buy goods, including medications for a simple flu as well as symptoms that they believe are attributable to the present crisis we are experiencing,” she said.
Last Dec. 31, the DOH said there was already a “high possibility” of local transmission of Omicron variant as it confirmed the detection of 10 new Omicron cases, 3 of which are local infections. The Philippines has so far detected 14.
‘Don’t panic’
Vergeire told Teleradyo on Jan. 5 that while Unilab Inc. confirmed the temporary shortage, it was already accelerating replenishment, “so we don’t need to panic,” explaining that if there’s lack of supply of some brands, “there are alternatives”.
READ: DOH on paracetamol ‘shortage’: Don’t panic, alternatives equally effective
Some of the paracetamol and analgesic brands that are temporarily out of stock are Biogesic, Neozep, Rexidol, Decolgen, Bioflu, Alaxan, and Tuseran.
The PHAP said paracetamol is available in different dosages, combination products and hundreds of brands. “Alternative analgesics are also available,” it said, explaining that there were enough analgesics in its inventory.
The Encyclopedia Britannica defined analgesics as those that “relieve pain selectively without blocking the conduction of nerve impulses, markedly altering sensory perception, or affecting consciousness.”
The Makati Medical Center (MMC) said through the years, branded medicines “have gained a reputation of being more expensive” than the generic ones–80 percent to 85 percent–implying that they are of high quality and more effective.
However, this is not the case.
The MMC explained that branded medicines are the first of their kind, with a pharmaceutical firm often having to go through research, development, and clinical trials to make it available to the public.
“From the time of inception, the formulation of the medicine is kept secret. This ends when the registered patent expires. Only then can other medicine manufacturers file to create a generic version, with only a 3.5% difference in absorption from the branded,” it said.
Generic medicines, meanwhile, work in the same way as the branded ones since they have the same quality, strength, suggested dosage, intended use, route of administration, effects, and side effects.
The MMC explained that the low cost of generic medicines is because the manufacturers of these are usually not the ones who developed, lab-tested, and marketed the medicine.
Graphic by Ed Lustan
“They also did not have to pay patent fees for it–which means cheaper costs to manufacture and sell to the public,” it said. Here’s how the MMC compared branded and generic medicines:
Branded
- The first version of the medicine in the market which went through rigorous testing for efficacy.
- Though extremely rare, there may be a small chance that someone’s body absorbs and receives a branded medicine better.
- “Recall and loyalty is higher” because of the time spent on marketing and branding.
- Branded medicines are often more expensive.
Generic
- Generic medicines are the exact copy of their branded counterparts.
- These are often cheaper than the branded ones.
- It is more widely available because firms have access to the ingredients list.
- These are also made with the same standards for the branded medicines.
- It doesn’t have a distinct look.
- Occasionally, some people may not do as well with a generic drug—they may absorb it differently due to how the tablet is coated or made.
- It may not be advisable to get a generic version of narrow therapeutic index drugs, in which tiny differences in the dose or formulation may cause a reaction.
The PHAP explained that patients can consult doctors and pharmacists for alternatives when some brands of paracetamol are not available.
‘Don’t overstock’
The public was also asked by the PHAP to buy only what they need, saying people should not overstock because medicines have a limited shelf life or expiration date and that they should also consider others who may need medicines more.
While the Department of Trade and Industry (DTI) said there was enough supply and that deliveries were being expedited to replenish retail stock inventories, it asked the public to only buy what is necessary.
READ: No paracetamol shortage, but purchase limit eyed – DOH, DTI
Trade Secretary Ramon Lopez said the DTI is also asking pharmacies to limit the purchase of paracetamol. He also asked the public to file complaints against business owners who sell medicines, like paracetamol, above the Suggested Retail Price.

