US FDA leaning toward approving Moderna half-dose booster–Bloomberg News
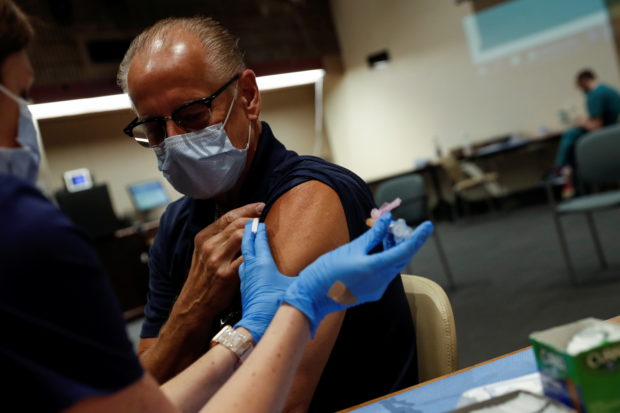
A registered nurse applies a dose of the Moderna COVID-19 vaccine to Sarasota Hospital worker Larry Hammers, 62, at the Sarasota Memorial Hospital in Sarasota, Florida, U.S., September 24, 2021. REUTERS FILE PHOTO
The U.S. Food and Drug Administration (FDA) is leaning toward authorizing half-dose booster shots of the Moderna Inc COVID-19 vaccine, Bloomberg News reported on Tuesday, citing people familiar with the matter.
The FDA had been seeking information about the effectiveness of a full third dose of the Moderna vaccine, but is now ready to move forward and consider the half-dose booster Moderna has proposed, the report said.
Moderna and the FDA did not immediately respond to Reuters request for comment outside regular business hours.
Moderna on Sept. 1 submitted its application to the U.S. Food and Drug Administration seeking authorization for a booster shot.
The original Moderna vaccine contains 100-micrograms of mRNA in each shot. The company’s submission to regulators to authorize a half-dose booster would allow Moderna to produce more.