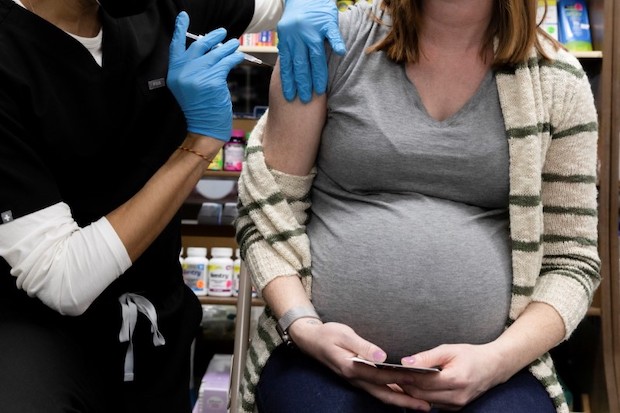
A pregnant woman receives a vaccine for the coronavirus disease (COVID-19) at Skippack Pharmacy in Schwenksville, Pennsylvania, US., Feb. 11, 2021. (REUTERS)
Pregnant women who get an mRNA vaccine against COVID-19 pass high levels of protective antibodies on to their babies, new research shows.
Doctors analyzed umbilical cord blood from 36 newborns whose mothers had received at least one dose of an mRNA vaccine from Pfizer-BioNTech or Moderna.
All 36 babies had high levels of antibodies that target the spike protein on the surface of the virus—and all of the antibodies could be traced to the mothers’ vaccinations.
The findings, reported on Wednesday in the American Journal of Obstetrics and Gynecology, indicate that “the antibodies that the mother is building to the vaccine are crossing the placenta and that’s likely to confer benefits for the infant after it’s born,” said coauthor Dr. Ashley Roman of NYU Langone Health in New York City.
It is not clear whether the timing of vaccination during pregnancy is related to antibody levels in the baby.
Roman said “We don’t know how long those antibodies stick around in the baby.”
But, she added, “the presence of these antibodies in the cord blood, which is the fetus’ blood, indicates that the baby also has potential to derive benefit from maternal vaccination.”
There are other recent studies on COVID-19 that also warrant further study and have yet to be certified by peer review.
‘Antibody map’
A new COVID-19 “antibody map” is helping researchers identify antibodies that will be able to neutralize the coronavirus even after it mutates, according to a report published on Thursday in Science Magazine.
Using hundreds of antibodies collected from COVID-19 survivors around the world, a global research team mapped out exactly where each antibody attaches to the spike protein on the virus surface, which it uses to break into cells and infect them.
The researchers looked for—and found—antibodies that target sites on the spike that are so important for the life cycle of the virus that it probably could not function without them.
Those sites are likely to remain targets for vaccines or treatments even when the virus mutates.
“If you are making an antibody cocktail, you’d want at least one of those antibodies in there because they are probably going to maintain their efficacy against most variants,” coauthor Kathryn Hastie of the La Jolla Institute for Immunology in California said in a news release.
J&J vaccine efficacy
A study on the Johnson & Johnson vaccine which compared nearly 400,000 Janssen vaccinees with 1.52 million unvaccinated people of similar ages, genders and health issues showed that the one-dose vaccine was 79-percent effective at preventing confirmed coronavirus infections and 81-percent effective at preventing COVID-19 hospitalizations.
The company cited on Tuesday those findings as also contained in a report posted on the medRxiv website ahead of peer review.
There was no evidence of reduced effectiveness during the study’s period of March to late July—amid the rise back then of the highly contagious Delta variant of the coronavirus, the company noted.
Preliminary data from a separate study suggest a second booster dose given about two months after the first shot increased the vaccine’s efficacy to 94 percent in the United States against moderate to severe forms of COVID-19.
When Johnson & Johnson announced its findings, half the people in the study had received the second shot at least 36 days prior.