COVID test kits expire amid low testing last year
FILE PHOTO: Persons identified for contact tracing in connection to COVID-19 patients undergo swabbing procedures inside UP-Philippine General Hospital, Manila. INQUIRER PHOTO/LYN RILLON
MANILA, Philippines—Information emerging from the Senate blue ribbon committee investigation of COVID spending by the Procurement Service of the Department of Budget and Management (PS-DBM) and Department of Health (DOH) said at least P550 million worth of testing kits had expired last year without being used.
A committee member, Sen. Francis Pangilinan, presented documents showing that Pharmally Pharmaceutical Corp.—a foreign-owned firm now at the center of the ongoing Senate investigation of P8.7 billion in contracts for COVID supplies—delivered 4,800 kits on May 2, 2020 and 3,200 on May 4, 2020.
Citing documents, Pangilinan said inspection reports would show that the test kits were manufactured on April 5, 2020 and had an expiry date of Oct. 5, 2020—a shelf life of just six months.
It was much shorter than what the DOH had required—24 to 36 months, which had been confirmed by Health Secretary Francisco Duque III but explained by him as benchmarks set prior to the pandemic.
Presented during the blue ribbon committee hearing was a letter by Assistant Health Secretary Nelson Santiago addressed to then PS-DBM chief Christopher Lloyd Lao dated Dec. 7, 2020 which confirmed that 7,925 test kits had expired without being used.
Article continues after this advertisementThese would have been good for at least 371,794 COVID tests last year.
Article continues after this advertisementREAD: ‘Gravity of waste’: Nearly P2B lost due to near-expiry, expired test kits — Pangilinan
READ: P550 million lost in expired COVID-19 test kits from Pharmally
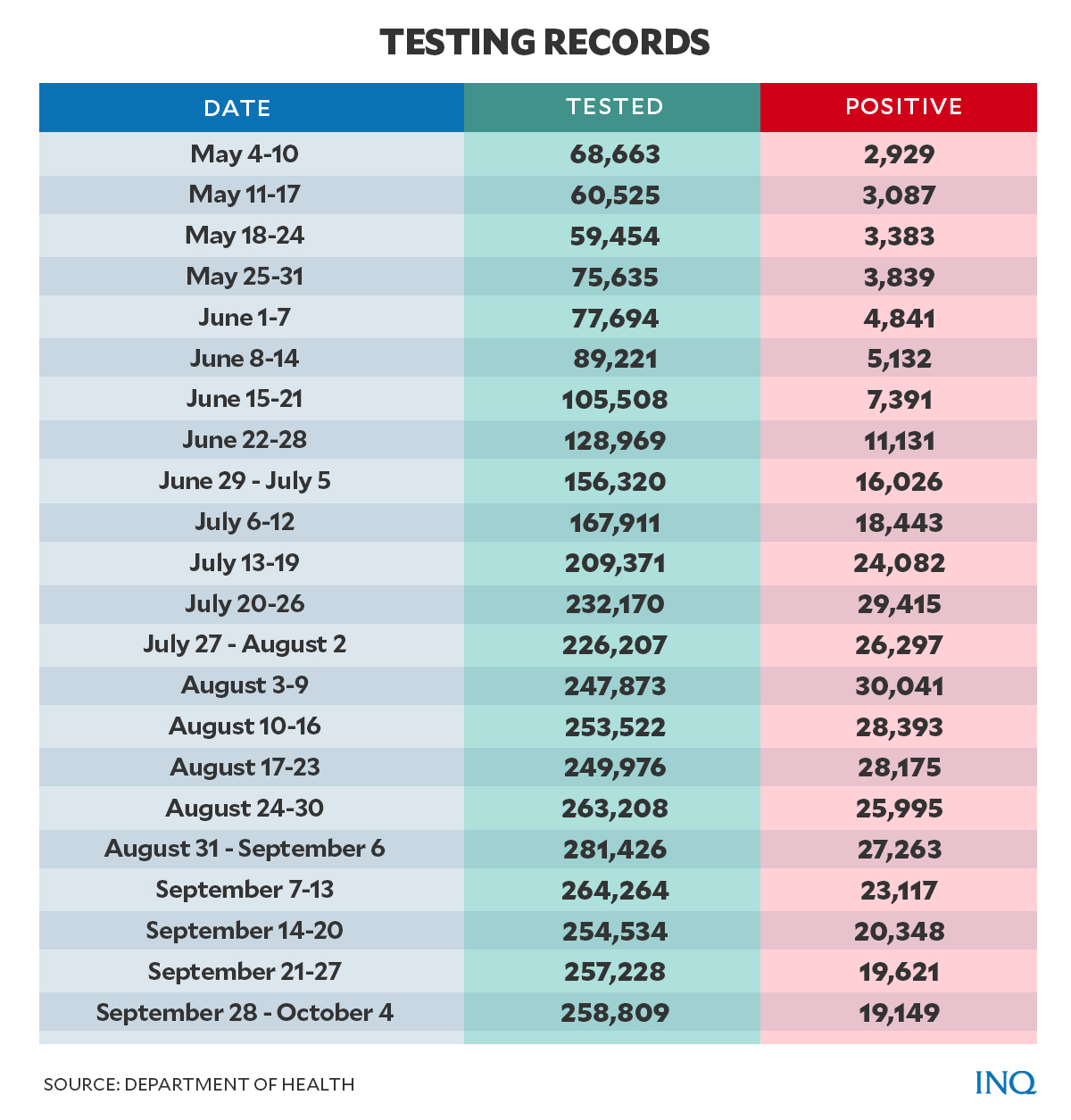
Between May and early October 2020, the country’s testing was still low despite thousands of test kits being delivered.
Low testing
According to the DOH data tracker, weekly testing nationwide from May 2020—when the COVID test kits were reportedly delivered—to October 2020—before the kits expired—was between 59,000 and 260,000.

Graphic by Ed Lustan
For comparison, weekly testing in 2021 from August 30 to Sept. 5 was 574,980 and 566,281 from Sept. 6 to 12.
The DOH said it “did not disregard” the shorter shelf life of the test kits bought by PS-DBM from Pharmally and which employ the reverse transcription-polymerase chain reaction (RT-PCR) technology, said to be the gold standard in COVID testing.
Health Undersecretary Charade Mercado-Grande, of the DOH’s regulation team, said the DOH was able to use the test kits delivered in 2020.
“The COVID-19 RT-PCR test kits with six months shelf life are not near expiry, as that was the standard shelf life of those novel diagnostic test kits at the time,” she said in a statement.
“Additionally, test kits are fast-moving stocks that have to be used immediately since we are in a pandemic. Our COVID-19 laboratories were able to test our countrymen) using these procured test kits,” she added.
READ: DOH denies Pharmally test kits purchased with six-month shelf life are nearing expiry
In May 2020, based on guidelines set by DOH, the following persons were subjected to COVID-19 tests:
- All symptomatic individuals
- Individuals arriving from overseas
- All close contacts of COVID-19 patients (contact tracing)
- Those who tested positive in rapid antibody test results
READ: Palace: No COVID-19 mass testing, only expanded targeted testing
Amid calls for mass testing last year, Duque admitted that there has been no mass testing conducted since the COVID-19 pandemic reached the Philippines.
READ: Duque admits no COVID-19 mass testing ever conducted since outbreak
Around the same time, DOH clarified that the country’s supply of test kits was “enough and sufficient for everybody.”
However, the government had a problem procuring other materials needed for testing, like reagents and other laboratory equipment.
No choice
In defense of the DOH, the Food and Drug Administration (FDA) explained that the health department had “no choice” but purchase test kits that had a shelf life of six months.
FDA Director General Eric Domingo clarified that all test kits made commercially available early in 2020 had a six-month shelf life.
“That means that the test kit for RT-PCR for COVID has very new technology. So if the technology is newly developed, we only learn its shelf life after it has been manufactured for a long time,” Domingo said.
READ: DOH had no choice but to buy test kits with a six-month shelf life – FDA chief
Domingo’s explanation was reflected in an earlier statement by DOH.
“Back then, real-time RT-PCR test kits that were available in the market had a shelf life of only six months. This was due to manufacturers having no data on whether their test kits would be stable and useful beyond six months,” it explained.
Graphic by Ed Lustan
The health department said the test kits used to detect SARS Cov2, the virus that causes COVID-19, which is a new disease, were developed only last year.
“The DOH acknowledged that these products have a short shelf-life and accepted the deliveries with this limitation. The deliveries of test kits were then requested on a staggered basis dependent on the consumption of the country,” it added.
Flagged earlier
The issue on test kits with only six-month shelf life was already flagged in 2020.
In December that year, Baguio City Mayor Benjamin Magalong claimed that a private diagnostic center in the city received nearly-expired 50,000 RT-PCR test kits from the Research Institute for Tropical Medicine (RITM) in October.
The DOH and RITM, on the other hand, denied that they released expired COVID-19 test kits to any laboratory in the country.
“In response to reports of alleged distribution of expired RT-PCR test kits by the RITM to Baguio City, the DOH and RITM clarify that based on delivery records and documents, no expired testing kits were released to any laboratory,” the DOH said in a statement at the time.
In the same statement, DOH explained that the testing kits have a shelf life of six months from the date of manufacturing, which is a shorter period compared to medicines that have three to five years of shelf life.
It added that “machine compatibility, technical issues, low testing referral count, and availability of supplies also contribute to low consumption of the COVID test kits which in turn increase the likelihood of expiry before use.”
TSB
For more news about the novel coronavirus click here.
What you need to know about Coronavirus.
For more information on COVID-19, call the DOH Hotline: (02) 86517800 local 1149/1150.
The Inquirer Foundation supports our healthcare frontliners and is still accepting cash donations to be deposited at Banco de Oro (BDO) current account #007960018860 or donate through PayMaya using this link.














