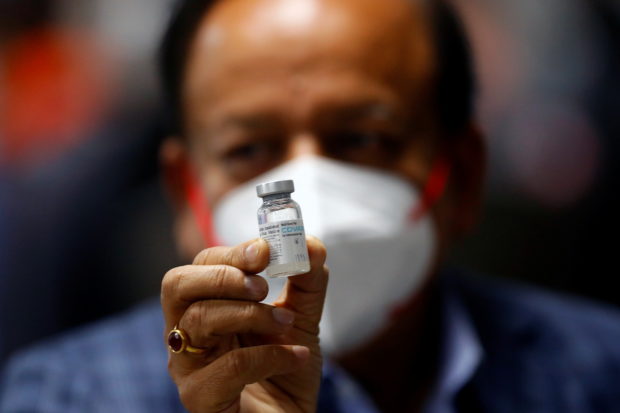
FILE PHOTO: Indian Health Minister Harsh Vardhan holds a dose of Bharat Biotech’s COVID-19 vaccine called COVAXIN, during a vaccination campaign at All India Institute of Medical Sciences (AIIMS) hospital in New Delhi, India, January 16, 2021. REUTERS/Adnan Abidi
MANILA, Philippines — India’s Bharat Biotech may now ship its vaccines against COVID-19 to the country as it has secured a full emergency use authorization (EUA) from the Food and Drug Administration (FDA).
Eric Domingo, FDA director general, said the full EUA was given to Bharat Biotech’s Covaxin after it submitted a good manufacturing practice certificate as required by the FDA.
Domingo explained that without this certificate, it would be unable to bring the vaccine into the county.
“But they submitted it last week, so now they could import and use [the vaccine] in the country,” Domingo said at an online briefing.
The private sector had ordered 8 million doses of Bharat’s Covaxin. —Leila B. Salaverria