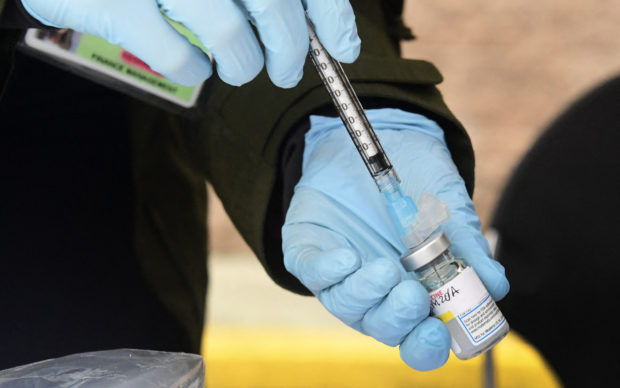
In this file photo, Registered Nurse Emily Enos loads the Moderna COVID-19 vaccine into a syringe ahead of the distribution of vaccines to seniors above the age of 65 who are experiencing homelessness at the Los Angeles Mission, in the Skid Row area of Downtown Los Angeles, California on February 10, 2021, as the fight against the coronavirus pandemic continues. (Photo by Frederic J. BROWN / AFP)
WASHINGTON — US biotech firm Moderna said Monday it was seeking conditional approval for use of its COVID-19 vaccine on teens in Europe and Canada, in what will assuredly be a boost for inoculation campaigns.
“We are encouraged that the Moderna COVID-19 vaccine was highly effective at preventing COVID-19 and SARS-CoV-2 infection in adolescents,” Moderna CEO Stephane Bancel said in a statement, adding that the firm also planned to file for emergency approval with the US Food and Drug Administration.