DOH to apply for EUA of Sinopharm’s COVID-19 vaccine

MADE IN CHINA The Beijing Institute of Biological Products claims the Sinopharm vaccine is 79 percent effective. —REUTERS
MANILA, Philippines — The Department of Health (DOH) is set to file on Monday an application for emergency use authorization (EUA) for the COVID-19 vaccine developed by China’s state-owned drugmaker Sinopharm.
This is after the World Health Organization (WHO) approved the vaccine for emergency use, making it the first shot developed by a non-Western country to secure WHO approval.
“Ngayong umaga, ang DOH mag-aapply ng EUA sa Food and Drug Administration for Sinopharm dahil mayroon nang EUL (emergency use listing) na nilabas ng WHO kahapon o nung makalawa,” Duque said in an interview on ABS CBN’s TeleRadyo.
(This morning, the DOH will apply for an EUA before the FDA for Sinopharm because it already has an EUL from the WHO.)
An EUA would allow a vaccine under development to be used in the country.
Article continues after this advertisementThe FDA earlier said there is no pending EUA application for the Sinopharm vaccine but three companies have so far expressed interest to apply.
Article continues after this advertisementPresident Rodrigo Duterte earlier got inoculated with the donated Sinopharm vaccine sans an EUA.
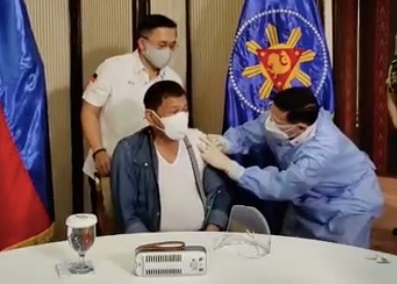
Health Secretary Francisco Duque III administers Sinopharm’s COVID-19 vaccine to President Rodrigo Duterte on Monday, May 3, 2021, in Malacañang. (Screenshot from Sen. Bong Go’s Facebook livestream)
Duterte later apologized and asked China to recall its donation.
Malacañang earlier said the President would still get his second dose of the Chinese vaccine.