DOH drafting guidelines on mixed-use vaccination — FDA
MANILA, Philippines — The Department of Health (DOH) is already drafting guidelines to allow a person to receive different brands of coronavirus vaccine for first and second dose, an official of the Food and Drug Administration (FDA) said Thursday.
FDA Director General Eric Domingo said this following President Rodrigo Duterte’s request for China to recall its 1,000-dose donation of Sinopharm vaccine, the same vaccine that he had himself inoculated Monday night. Incidentally, Sinopharm has yet to secure emergency use authorization from FDA up to this time.
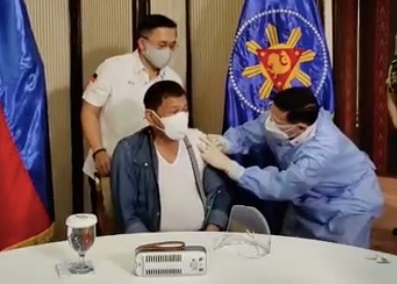
Health Secretary Francisco Duque III administers Sinopharm’s COVID-19 vaccine to President Rodrigo Duterte on Monday, May 3, 2021, in Malacañang. (Screenshot from Sen. Bong Go’s Facebook livestream)
Addressing queries on what would happen to those who had been jabbed with Sinopharm vaccine as first dose, Domingo said that the DOH is now studying the possibility of mixed-use vaccination, especially for vaccinees who experienced severe allergic reactions in their first dose, and have been apprehensive about receiving a second dose of vaccine from the same brand.
“Gumagawa ng guidelines ngayon ang DOH kung paano ‘yung interchangeability o mixing ng vaccines in case the second dose cannot be given na identical doon sa first dose,” he said in a Laging Handa briefing.
(The DOH is drafting guidelines on the interchangeability or mixing of vaccines in case the second dose of the same vaccine brand cannot be administered.)
Duterte was inoculated last Monday, May 3, with the China-made COVID-19 vaccine, but soon after, the President apologized for receiving a dose of the Sinopharm vaccine.
Palace earlier said Duterte is covered under the compassionate use permit issued by the FDA to the Presidential Security Group.