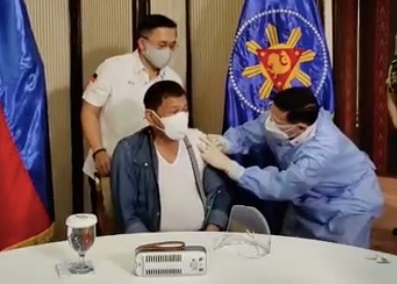
Health Secretary Francisco Duque III administers Sinopharm’s COVID-19 vaccine to President Rodrigo Duterte on Monday, May 3, 2021, in Malacañang. (Screenshot from Sen. Bong Go’s Facebook livestream)
MANILA, Philippines — President Rodrigo Duterte was inoculated Monday, May 3, against COVID-19 using the vaccine developed by China’s Sinopharm, which has yet to receive an emergency use authorization (EUA) from the Food and Drug Administration (FDA).
Health Secretary Francisco Duque III was to administer the vaccine to Duterte as seen in a livestream on the Facebook page of the President’s long-time aide, Senator Christopher “Bong” Go. The footage was abruptly stopped just as when Duque was to supposedly inject the vaccine.
“I feel good and I have been expecting this shot, vaccination a long time ago,” Duterte said before his supposed vaccination, which purportedly happened in Malacañang.
Duterte said his doctor took “a long time to make the assessment” before Sinopharm was chosen as the appropriate vaccine for him.
“Sinopharm itong tinuturok sa akin,” he added.
In a subsequent statement, presidential spokesperson Harry Roque confirmed Duterte received Monday night his first dose of the Sinopharm vaccine.
“His first dose was covered by the Compassionate Use Permit issued to the PSG hospital by the FDA,” he explained.
Malacañang had been saying that Duterte prefers to get the COVID-19 vaccine developed by Sinopharm.
To date, Sinopharm’s EUA application remains pending before the FDA.
In February, however, the FDA granted compassionate use of 10,000 doses of the Sinopharm vaccine for members of the Presidential Security Group (PSG).
The issuance of a compassionate use license means the vaccines can be administered even without securing a EUA that is required for COVID-19 vaccines to be legally administered in the country.
No detailed efficacy data of Sinopharm’s vaccine has been made public, but its developer said the jab has 79.34% efficacy to prevent people from contracting the disease based on interim data.