India’s COVID-19 vaccine Covaxin granted emergency use authorization
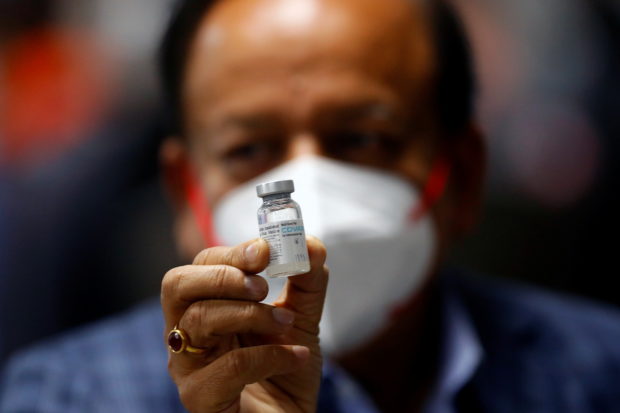
Indian Health Minister Harsh Vardhan holds a dose of Bharat Biotech’s COVID-19 vaccine called COVAXIN, during a vaccination campaign at All India Institute of Medical Sciences (AIIMS) hospital in New Delhi, India, January 16, 2021. REUTERS/Adnan Abidi
MANILA, Philippines — The Food and Drug Administration (FDA) has granted Covaxin, the COVID-19 vaccine developed by India’s Bharat Biotech, an emergency use authorization (EUA).
FDA Director-General Eric Domingo on Tuesday said the country’s experts, upon studying the drugmaker’s data on its clinical trials, found that the benefit of administering the Covaxin “outweighs its risks.”
“They initially applied with us noong Jan. 22. Noong March 9 binigay po nila sa atin ‘yung kanilang clinical trial data at ‘yun ang tiningnan ng ating experts and they decided that the benefit of this outweighs the risks,” he said in a Laging Handa public briefing.
Bharat Biotech still has to submit another document, a “certain certificate,” before the Covaxin can be imported into the country, Domingo said.
Article continues after this advertisementIn March, India’s ambassador to the Philippines Shambhu Kumaran said the Philippines and India are in talks for the supply of eight million or more doses of Covaxin.
Article continues after this advertisementPresidential adviser for entrepreneurship Joey Concepcion earlier said the private sector may start receiving a million doses of Covaxin in April.
Bharat Biotech earlier revealed that preliminary data from phase 3 trials for the Covaxin showed it has an efficacy rate of 81 percent.
Aside from Covaxin, FDA has also recently granted an EUA to the COVID-19 vaccine of Johnson & Johnson.
At present, there are six vaccines that have EUA in the country. Domingo said there is no pending EUA application since Sinopharm and Moderna have yet to submit their respective applications.