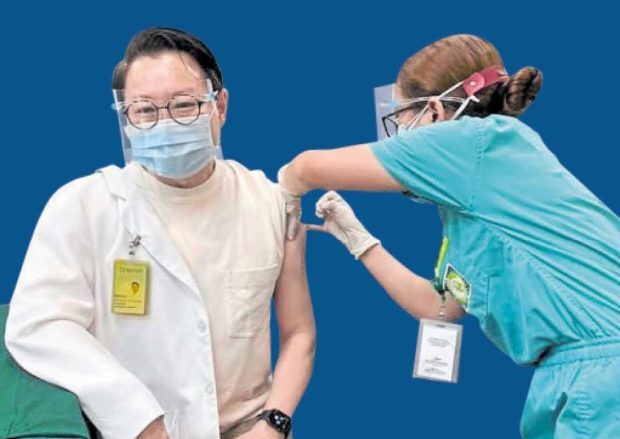
OK WITH SINOVAC Dr. Benjamin Co, a 62-year-old infectious disease specialist at Asian Hospital and Medical Center, gets the first dose of CoronaVac on March 3 to encourage more health-careworkers to get the China-made vaccine made by Sinovac Biotech, or any vaccine for that matter, to protect the front-line medical
workforce. —PHOTO FROM DR. BENJAMIN CO’S SOCIAL MEDIA ACCOUNT
MANILA, Philippines — Pediatric cardiologist Olympia “Pia” Malanyaon and her husband, otorhinolaryngologist Jose Malanyaon Jr., were completely sold on the idea of getting vaccinated — whatever the brand and what was first available.
Working at front-line government hospitals, Malanyaon said she and her husband were aware that getting exposed to the coronavirus was just a matter of time.
“We see patients at the clinic, we make rounds at the hospitals with exposure to possible ‘silent’ carriers. The sooner we get the vaccines and develop antibodies, the better,” she said.
The Philippines kicked off its COVID-19 vaccination program on March 1, with the initial 600,000 doses of CoronaVac donated by China and produced by the Chinese pharmaceutical company Sinovac Biotech.
At the top of the priority list of people to be inoculated are the front-line health-care workers.
The Food and Drug Administration (FDA) has granted emergency use authority to Sinovac, AstraZeneca, Pfizer of the United States and Russia’s Sputnik V. The Pfizer and Sputnik V shots have not been delivered. But there’s a caveat, as far as CoronaVac is concerned.
The FDA said CoronaVac was not exactly the best for health workers who directly treat COVID patients (with just 50.4-percent efficacy for this particular group), those who are 60 and older, and those with existing health issues.
The FDA’s advise stirred public debate at a time when there was no other vaccine available for health-care workers.
It would be days after CoronaVac was delivered that the shots from AstraZeneca, developed by the University of Oxford, arrived in the Philippines from COVAX, the international vaccine pool.
Many in the health sector who had listed up to get the Pfizer shots were unhappy and felt that they were being forced to receive the “less efficacious” vaccine.
Hesitancy
Vaccine hesitancy and brand preference set in.
After some discussions within the National Immunization Technical Group, a panel of disease and vaccine experts, the government went ahead with the CoronaVac rollout in the hospitals and left the decision—whether to get it or defer inoculation until their preferred brand arrives—to the medical workers themselves.
But for Malanyaon, 64, and her husband, 65, it wasn’t really a difficult decision to make.
Malanyaon got her first CoronaVac shot on March 3 at the Philippine General Hospital (PGH), the same day her husband got his at East Avenue Medical Center. The couple will get their second shot on Holy Wednesday, March 31.
Getting CoronaVac also wasn’t a big concern for infectious disease expert Benjamin Co, 62, of the Asian Hospital and Medical Center.
The doctors took the shot, saying the best vaccine is the one in the arm.
Several other senior doctors took CoronaVac shots, including ophthalmologist Minguita Padilla, the president of the Eye Bank of the Philippines who actively campaigns against vaccine mistrust.
Others at PGH and East Avenue also did, after signing an “informed consent.”
No regrets
Both Malanyaon and Co said they didn’t regret it.
Besides, Malanyaon said, senior doctors usually saw patients who had been prescreened for COVID-19 and by appointment or through online consultations.
Senior physicians were at a relatively lower risk of getting infected than residents and nurses who routinely worked the wards.
Co said the FDA made its recommendation based on the available and peer-reviewed data the drug company submitted to the regulatory agency.
In the same clinical trial done exclusively among health-care workers in Brazil, CoronaVac did show a lower efficacy in preventing mild cases, but, on the other hand, prevented participants from developing severe illness which was another “vaccine endpoint,” he said.
“A vaccine is a vaccine,” Co said.
He said that aside from his personal protection, Co also wanted to inspire vaccine confidence among his colleagues.
Currently, the government is discussing the use of CoronaVac on the elderly and next vulnerable population once it begins the inoculation of the general public.
Presidential spokesperson Harry Roque said they would ask the drug company to provide more data on the vaccine in order to expand the coverage of the FDA-issued authority.
Malanyaon said CoronaVac used an inactivated or dead virus, a time-tested platform or process similarly used in the development of many vaccines for other diseases.
The cardiologist said any vaccine, as long as approved by the FDA, was safe. More than anything else, people should trust science, she said.