Trials for 3 COVID-19 vaccines show promise but much more work still needed, say experts

A general view is pictured of the offices of British-Swedish multinational pharmaceutical and biopharmaceutical company AstraZeneca PLC in Macclesfield, Cheshire on July 21, 2020. Photo by Paul ELLIS / AFP
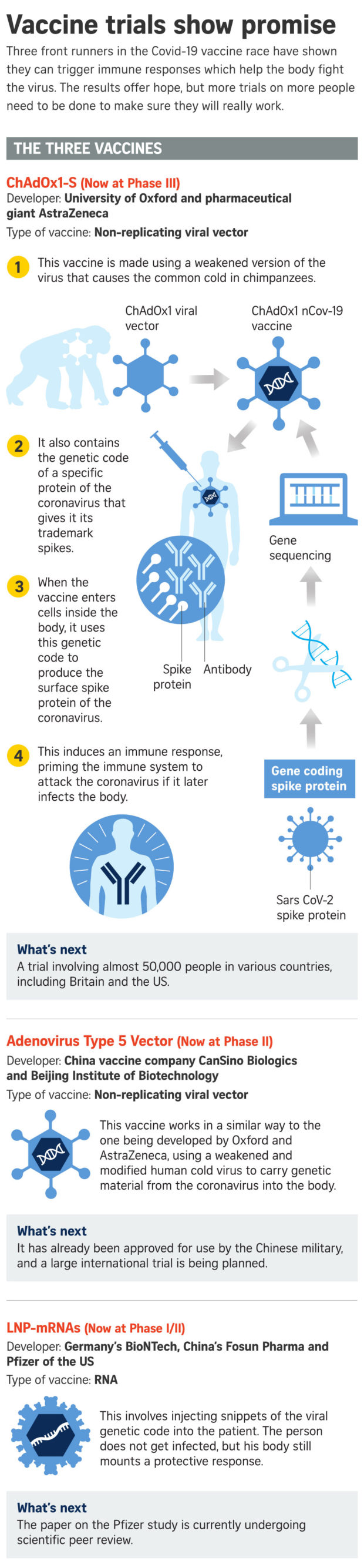
SINGAPORE — The global fight against Covid-19 received a boost on Monday with the release of encouraging findings from human trials of three coronavirus vaccines, including a closely watched one being developed by Oxford University.
The results showed that the vaccines being tested did not cause any dangerous side effects, and that they could coax a protective response from the human body.
But experts said that while the results were encouraging, much more work is still needed to plug remaining gaps in knowledge before a vaccine can be made commercially available.
Speaking at a media conference on Monday, Dr Michael Ryan, executive director of the World Health Organization’s health emergencies program, noted that the findings were from trials in their early stages and the data is very new.
“We now need to move into larger-scale, real-world trials,” he said. “But it is good to see more data, and more products moving into this very important phase of vaccine discovery, and we congratulate our colleagues for the progress they have made.”
The latest findings were from early phases of clinical trials for vaccines being developed by Oxford University and multinational drugmaker AstraZeneca; CanSino Biologics and China’s military research unit; and German biotech company BioNTech and US drugmaker Pfizer.
All three studies – two were published in medical journal The Lancet, while the Pfizer study was published as a pre-print pending peer review – found that the vaccine candidates could stimulate the body to produce immune system “soldiers”.
These soldiers, such as antibodies or T-cells, help the body fight off invading pathogens.
Professor Stephen Evans from the London School of Hygiene and Tropical Medicine, noted that for the Oxford trial, the immune responses measured in the blood, and the absence of serious harms indicate there is a possibility of an effective vaccine against Covid-19.
The Oxford vaccine had prompted an antibody and a T-cell response – both of which may be important in the protection against Covid-19.
However, Prof Evans also noted that there were some gaps.
“It does not yet show that the disease is reduced or prevented, and this will not be easy to show until Phase III trials have been completed in settings where the (virus) is circulating at a high rate and people are getting clinical and severe disease,” Prof Evans was quoted as saying in a CNN report.
Clinical development is a three-phase process, said the US Centers for Disease Control and Prevention on its website.
In Phase I, small groups of people receive the trial vaccine, and this is expanded in Phase II to include people who have characteristics, such as age and physical health, similar to those for whom the new vaccine is intended.
In Phase III, the vaccine is given to thousands of people and tested for efficacy and safety.
In an update on Monday, AstraZeneca said late-stage Phase II/III trials are currently under way in Britain, Brazil and South Africa and are due to start in the United States.
“Trials will determine how well the vaccine will protect from the Covid-19 disease and measure safety and immune responses in different age ranges and at various doses,” the company noted.
The findings from the three trials follow the publication last week of the results of Moderna’s vaccine trial, which showed similarly promising early results.
Moderna’s vaccine is similar to the Pfizer one in that it also uses a messenger RNA platform.
There are currently more than 150 vaccine candidates being studied worldwide.
At a separate press conference last week, Professor Leo Yee Sin, executive director of Singapore’s National Centre for Infectious Diseases, noted that most vaccines can take seven to 15 years to become commercially available, depending on the pathogen.
“For Covid-19, people are looking at 12 to 18 months, so this is still a space that we are watching very closely,” she said then.
“But there are a lot of international collaborations and efforts being put in to see whether we can see an effective, usable, deployable vaccine for the human race around the world.”
For more news about the novel coronavirus click here.
What you need to know about Coronavirus.
For more information on COVID-19, call the DOH Hotline: (02) 86517800 local 1149/1150.
The Inquirer Foundation supports our healthcare frontliners and is still accepting cash donations to be deposited at Banco de Oro (BDO) current account #007960018860 or donate through PayMaya using this link.