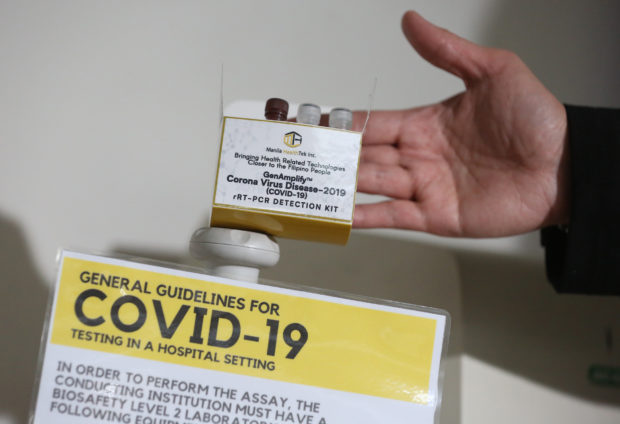
FIELD TESTING TODAY: The locally developed GenAmplify kit that will be field tested beginning on March 16. (Photo by NIÑO JESUS ORBETA / Philippine Daily Inquirer)
MANILA, Philippines — Malacañang again assured the public on Sunday that the government had sufficient funds for diagnostic kits for the coronavirus disease-19 (COVID-19).
“We wish to assure our countrymen that the Office of the President is providing the needed funds of the Department of Health, Department of Science and Technology, and UP Manila’s National Institutes of Health in the production of the diagnostic kits for the COVID-19 tests,” presidential spokesperson Salvador Panelo said in a statement.
He also dismissed “false narratives being circulated in the social media that the national government is short of funds to address the national health emergency, thereby creating apprehension and panic among the populace.”
Panelo issued the joint statement on Sunday with Science and Technology Secretary Fortunato dela Peña, who also denied that the Department of Science and Technology was seeking donations for test kits.
“DOST categorically states that it is not in any way involved in the call by some groups for donations regarding access to test kits. Sufficient funds are available,” he said.
The two officials issued the statement after outdated reports indicated that the DOH Research Institute for Tropical Medicine only had 2,000 test kits in their inventory, but Health Secretary Francisco Duque III said on Saturday that the government would soon have 3,500 more kits.
Moreover, scientists from the University of the Philippines have developed a cheaper test kit, the GenAmplify COVID-19 detection kit, and 6,000 test kits also have already been manufactured by Manila HealthTek Inc., which can produce 200 kits a week, said UP President Danilo Concepcion.
Panelo said Manila Healthtek was “prepared to do the production of bigger quantities required to respond to the present crisis, in addition to its present inventory once the imported raw materials arrive.”
For his part, dela Pena distanced the DOST from the call for donations made by some groups who would want more funding for the production of test kits.
According to Dr. Eric Domingo, director-general of the Food and Drug Administration, the GenAmplify test kits are set to start field testing on March 16 and will be available soon.
Aside from the GenAmplify kits, only the test kits at the RITM, which were donated by the World Health Organization, are approved by the FDA for local use. No company has submitted the requirements for FDA certification and Domingo warned against their use.
“We cannot vouch for its safety and efficacy by merely accepting the stated claims of a testing kit without the proper regulatory certification from the country of origin and a reliable national regulation agency,” Domingo said.
—With a report from Tina G. Santos